Clopidogrel hydrogensulfate composition and preparation method thereof
A technology for clopidogrel hydrogen sulfate and pharmaceutical preparations, applied in the field of medicine and biology, can solve problems such as poor stability, stickiness, unsuitability for industrial production, etc., and achieve the effects of improved stability and easy storage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
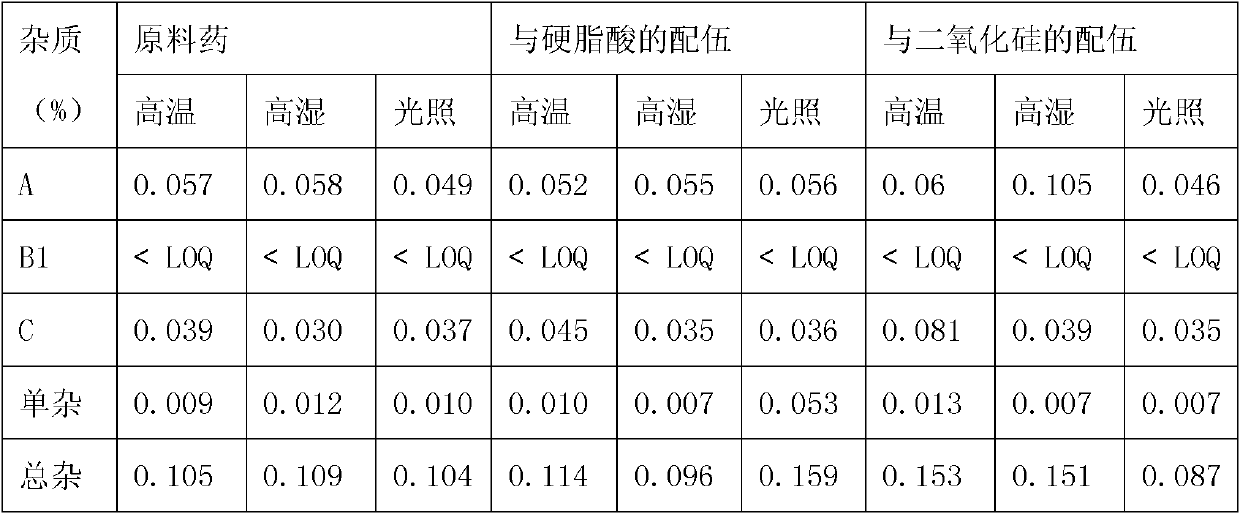
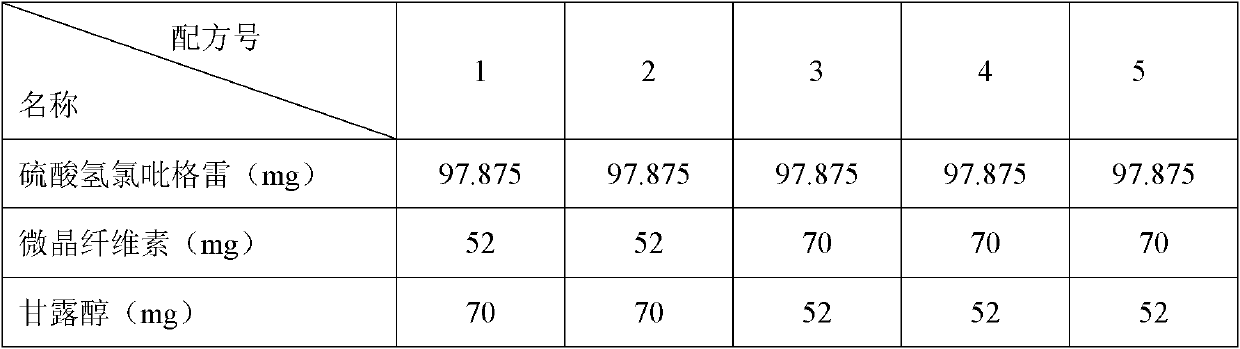
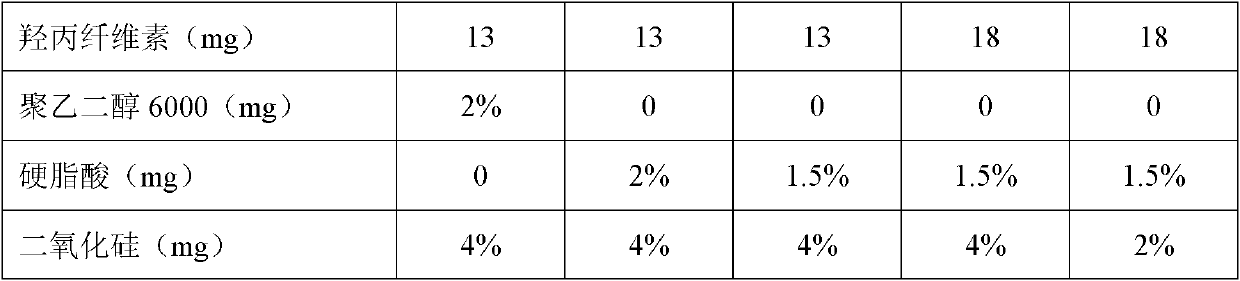
Image
Examples
Embodiment 1
[0104] formula:
[0105] Clopidogrel bisulfate 97.875 g
[0106] Microcrystalline Cellulose 70 g
[0107] Mannitol 52 g
[0108] Hypromellose 18 g
[0109] Stearic acid 3.5 g
[0110] Silica 9.3 g
[0111] Preparation method:
[0112] 1) Pass clopidogrel bisulfate, microcrystalline cellulose, mannitol, and hydroxypropyl cellulose through a 60-mesh sieve for subsequent use; pass stearic acid and silicon dioxide through a 100-mesh sieve for subsequent use; Clopidogrel, microcrystalline cellulose, mannitol, and 2 / 3 of the formula amount of hydroxypropyl cellulose were added and mixed in equal amounts, passed through a 16-mesh sieve with a dry preparation machine to granulate, and set aside; the formula amount was weighed separately The hydroxypropyl cellulose of stearic acid, silicon dioxide and 1 / 3 formula quantity are added in the granule of above-mentioned dry granule, mix well, detect the content of intermediate with high performance liquid chromatography; 2) tabletting...
Embodiment 2
[0114] formula:
[0115] Clopidogrel Bisulfate 80 g
[0116] Microcrystalline cellulose 80 g
[0117] Mannitol 55 g
[0118] Hypromellose 15 g
[0119] Stearic acid 2 g
[0120] Silica 10 g
[0121] Preparation method:
[0122] 1) Pass clopidogrel bisulfate, microcrystalline cellulose, mannitol, and hydroxypropyl cellulose through a 60-mesh sieve for subsequent use; pass stearic acid and silicon dioxide through a 100-mesh sieve for subsequent use; Clopidogrel, microcrystalline cellulose, mannitol, and 2 / 3 of the formula amount of hydroxypropyl cellulose were added and mixed in equal amounts, passed through a 16-mesh sieve with a dry preparation machine to granulate, and set aside; the formula amount was weighed separately The hydroxypropyl cellulose of stearic acid, silicon dioxide and 1 / 3 formula quantity are added in the granule of above-mentioned dry granule, mix well, detect the content of intermediate with high performance liquid chromatography; 2) tabletting 3) fil...
Embodiment 3
[0124] formula:
[0125] Clopidogrel Bisulfate 110 g
[0126] Microcrystalline cellulose 60 g
[0127] Mannitol 48 g
[0128] Hypromellose 20 g
[0129] Stearic acid 4 g
[0130] Silica 9 g
[0131] Preparation method:
[0132] 1) Pass clopidogrel bisulfate, microcrystalline cellulose, mannitol, and hydroxypropyl cellulose through a 60-mesh sieve for subsequent use; pass stearic acid and silicon dioxide through a 100-mesh sieve for subsequent use; Clopidogrel, microcrystalline cellulose, mannitol, and 2 / 3 of the formula amount of hydroxypropyl cellulose were added and mixed in equal amounts, passed through a 16-mesh sieve with a dry preparation machine to granulate, and set aside; the formula amount was weighed separately The hydroxypropyl cellulose of stearic acid, silicon dioxide and 1 / 3 formula quantity are added in the granule of above-mentioned dry granule, mix well, detect the content of intermediate with high performance liquid chromatography; 2) tabletting 3) fil...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com