Uvaria macrophylla bisamide derivative and preparation method and application thereof
A Ziyupan, bisamide technology, applied in the separation/purification of carboxylic acid amide, drug combination, pharmaceutical formula, etc., can solve the anti-cancer activity report of the chemical synthesis and preparation method of Ziyupan bisamide derivatives, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] Example 1 Preparation of Ziyupan bisamide (1)
[0069] Dissolve benzoic acid (3.7 g, 30 mmol) in 5 mL of thionyl chloride, add 2 drops (0.1 mL) of N,N-dimethylformamide, stir and reflux for about 1 hour, evaporate the solvent to obtain a solid. The solid was dissolved in 50 mL of dichloromethane, and a mixture of 1.32 g (15 mmol) butanediamine and 8 mL of triethylamine was added under cooling, and a solid appeared. After addition, stir at room temperature for 4 h. After the reaction was completed, 50 mL of water was added, filtered, and the filter cake was washed with 5% NaOH and 5% HCl, respectively. The washed solid was suction filtered, and the filter cake was dried to obtain compound (1).
[0070]
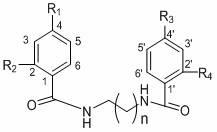
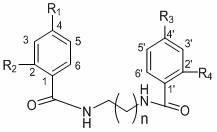
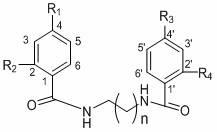
[0071] Compound (1) uvariadiamide: n=3, R 1 =R 2 =R 3 =R 4 =H.
[0072] Spectral data of Ziyupan bisamide: ESI-MS m / z 297 [M+H] + , the molecular weight is determined to be 296, and the molecular formula is C 18 h 20 N 2 o 2 . 1 H-NMR (600MHz, DMSO-d...
Embodiment 2
[0073] Example 2 Preparation of Ziyu Panxinsu (2)
[0074] Dissolve p-methoxybenzoic acid (4.5 g, 30 mmol) in 5 mL of thionyl chloride, add 2 drops of N,N-dimethylformamide, stir and reflux for about 1 hour, evaporate the solvent to obtain a solid. The solid was dissolved in 50 mL of dichloromethane, and a mixture of 1.32 g (15 mmol) butanediamine and 8 mL of triethylamine was added under cooling, and a solid appeared. After addition, stir at room temperature for 4 h. After the reaction was completed, 50 mL of water was added, filtered, and the filter cake was washed with 5% NaOH and 5% HCl, respectively. The washed solid was suction filtered, and the filter cake was dried to obtain compound (2).
[0075]
[0076] Compound (2) Violet Pansin (4,4 ′ -di-p-methoxybenzoyl-1,4-butanediamine): n=3, R 2 =R 4 = H, R 1 =R 3 =OCH 3 .
[0077] Ziyu Panxinin is a new natural product, the spectral data of Ziyu Panxinin: ESI-MS m / z 355.6 [M-H] - , with a molecular weight ...
Embodiment 3
[0078] Example 3 4, 4 ′ - Preparation of two p-nitrobenzoyl-1,4-butanediamine (3)
[0079] Dissolve p-nitrobenzoic acid (5 g, 30 mmol) in 5 mL of thionyl chloride, add 2 drops of N,N-dimethylformamide, stir and reflux for about 1 h, evaporate the solvent to obtain a light yellow solid . The yellow solid was dissolved in 50 mL of dichloromethane, and a mixture of 1.32 g (15 mmol) of butanediamine and 8 mL of triethylamine was added under cooling, and a solid appeared. After the addition was complete, stir at room temperature for 4 hours. After the reaction was completed, 50 mL of water was added, filtered, and the filter cake was washed with 5% NaOH and 5% HCl, respectively. The washed solid was suction filtered, and the filter cake was dried to obtain compound (3).
[0080]
[0081] Compound (3) 4,4’-di-p-nitrobenzoyl-1,4-butanediamine: n=3, R 2 =R 4 = H, R 1 =R 3 =NO 2 .
[0082] Its structure confirmed: white powder, ESI-MS: m / z 387.4 [M+H] + , 1 H-NMR (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com