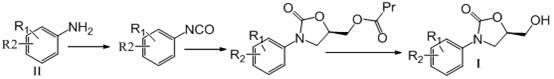
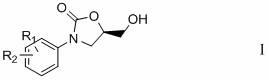
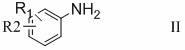Preparation method of 3-substituted phenyl-5-hydroxymethyloxazolidine-2-ketone
A technology of hydroxymethyloxazolidine and phenyl, which is applied in the field of preparation of pharmaceutical intermediates, can solve the problems of low utilization rate of carbon atoms, failure to meet the development trend, high production cost, etc., so as to improve the safety production factor and the price of raw materials Inexpensive, less environmental pollution effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056]
[0057] Add 29.4g (0.15mol) of 3-fluoro-4-(4-morpholinyl)aniline and (S)-3-chloro-1,2-propanediol into a 500ml four-necked flask equipped with stirring, condenser and thermometer 11.05g (0.1mol), 4.4g (0.11mol) of sodium hydroxide and 200ml of ethanol, stirred and heated to reflux for 12hrs.
[0058] The solvent was removed under reduced pressure, 200 ml of water was added, and stirred for 1 hr. After suction filtration, the aqueous solution of compound (2) was obtained, which can be directly used in the next step of cyclization reaction. After quantitative analysis, it contained 23.06g of (R)-(+)-3-(3-fluoro-4-morpholineanilino)-1,2-propanediol. Yield 85.4%.
[0059]
[0060] Stirring is equipped with in 1000ml, dropping funnel and thermometer add about 350ml (0.0854mol) of aqueous solution containing 23.06g compound (2) in example 1, 100ml dichloroethane. Under ice-salt bath cooling, control the liquid temperature at 0-5°C, and slowly add 120ml (0.11mol) of ...
Embodiment 2
[0067] Except that the amount of 3-fluoro-4-(4-morpholino)aniline is 19.6 g (0.1 mol), the others are the same as in Example 1. The aqueous solution of compound (R)-(+)-3-(3-fluoro-4-morpholineanilino)-1,2-propanediol was obtained, which can be directly used in the next step of cyclization reaction. After quantitative analysis, it contained 17.55g of (R)-(+)-3-(3-fluoro-4-morpholineanilino)-1,2-propanediol. The yield is 65%.
Embodiment 3
[0069] Except that ethanol is replaced with isopropanol, all the other are the same as embodiment one. The aqueous solution of compound (R)-(+)-3-(3-fluoro-4-morpholineanilino)-1,2-propanediol was obtained, which can be directly used in the next step of cyclization reaction. After quantitative analysis, it contained 19.04g of (R)-(+)-3-(3-fluoro-4-morpholineanilino)-1,2-propanediol. Yield 70.5%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com