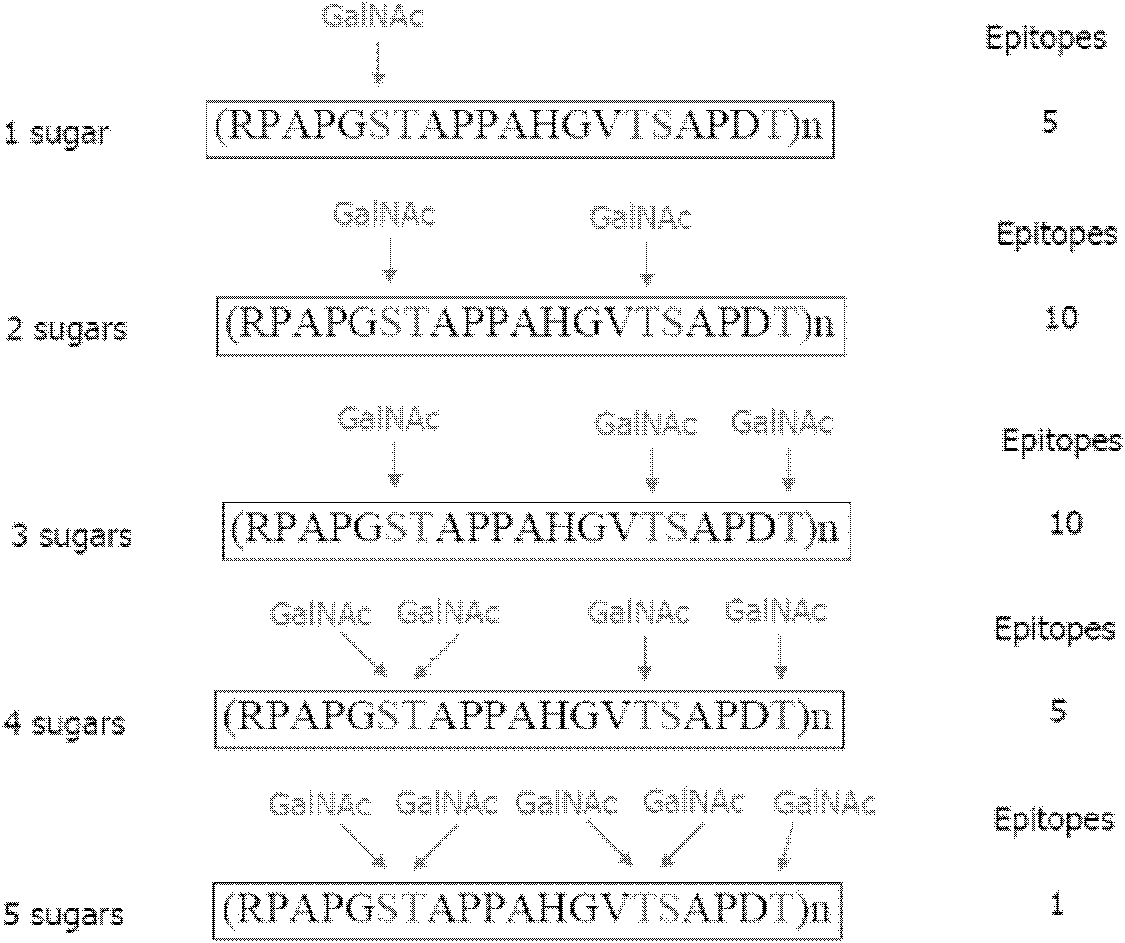Preparation method for monoclonal antibody library of tumor mucoprotein glycopeptide epitope
A monoclonal antibody, antigen-determined technology, applied in the fields of genetic engineering and immunology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1: Preparation of monoclonal antibody library for tumor mucin MUC1 glycopeptide epitope
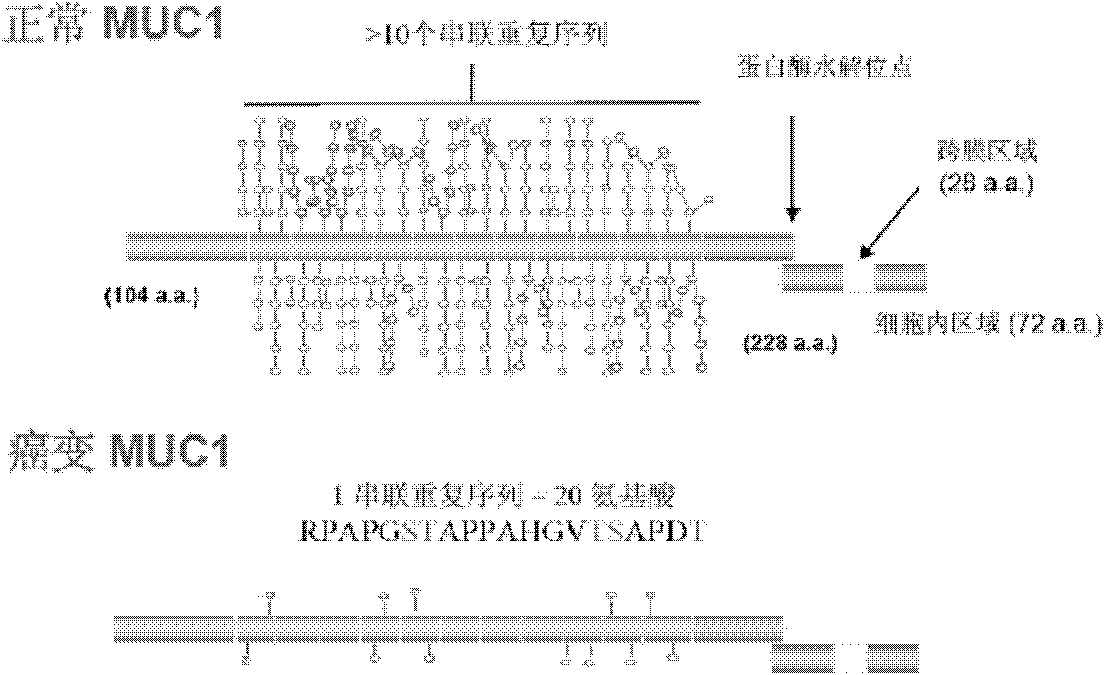
[0029] (1) According to the amino acid sequence of MUC1 ( figure 2 ), using the mathematical method of permutation and combination, deduced all possible glycopeptide sequences of MUC1 in tumorigenesis. Each MUC1 tandem repeat consists of 20 amino acids. Each repeat has 5 sites that can be modified by glycosylation. In this way, we can theoretically deduce the structure of possible glycopeptides. There are 5 with 1 sugar, 10 with 2 sugars, 10 with 3 sugars, 5 with 4 sugars, and 1 with 5 sugars. A total of 31 glycopeptides can be recognized by 31 different antibodies.
[0030] (2) Preparation of immunogen: In order to study the mucin on the surface of tumor cells, we designed a unique immunogen. We selected a tumor cell Jurkat expressing Tn antigen (A Mutant Chaperone Converts a Wild-Type Protein into a Tumor-Specific Antigen, Andrea Schietinger , et al. Science 31...
Embodiment 2
[0038] Purification and preparation of monoclonal antibody Clone 16: the hybridoma was inoculated in the peritoneal cavity of nude mice, and the ascites fluid was collected 10 days later, and purified with Protein A sepharose (Sigma, 2 ml) affinity chromatography column. The purified antibody was verified by SDS-PAGE to have no other protein bands except IgG heavy chain and light chain.
[0039] For the detection of the specific affinity of monoclonal antibody Clone 16 to glycopeptides: 2 μg / ml glycopeptides (in image 3 Glycopeptide 2u (represented) was conjugated with biotin and bound to a 96-well plate coated with streptavidin (1.5 μg / ml). Serially diluted Clone 16 antibody protein (protein concentration as Figure 6 shown) after binding to the glycopeptide, was detected with a goat anti-mouse secondary antibody. Secondary antibody with horseradish peroxidase was detected with DAB kit. The results showed that the antibody (Clone 16) had a high specific affinity for glyco...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com