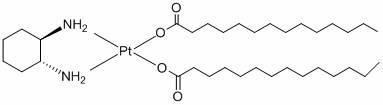
Preparation method of miriplatin hydrate
A technology of meplatin and a synthesis method, which is applied in the field of preparation of platinum antitumor drug meplatin, can solve the problems of chloroform being harmful to human body and long reaction period, and achieve the effects of avoiding injury, short production period and high yield.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
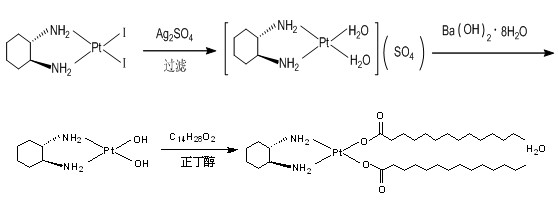
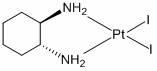
[0025] Under normal temperature and pressure, according to the liquid-solid mass ratio of 1g: 3ml, K 2 PtCl 4 Soluble in water, according to K 2 PtCl 4 : KI is 1g: 1.6g ratio to weigh KI, then according to the liquid-solid mass ratio of 1g: 5ml, dissolve KI in water and add the KI solution to K under the condition of dark stirring 2 PtCl 4 solution, after 30 minutes of reaction, press K 2 PtCl 4 : (1R, 2R)-1,2 cyclohexanediamine is 1g:0.27g Weigh (1R,2R)-1,2 cyclohexanediamine, according to the liquid-solid mass ratio of 1g:3ml, the (1R, 2R)-1,2 cyclohexanediamine was diluted with water and added to the solution, reacted for 2 hours and filtered to obtain a yellow precipitate, washed the filter cake with water for 3 times and when the filtrate was neutral, washed the filter cake with absolute ethanol for 2 times, Then dry at 70°C to obtain PtC with purity ≥98% 6 h 14 N 2 I 2 .
[0026] See accompanying drawing technological process. Weigh 5.63 g of cycloplatinum, a...
Embodiment 2
[0030] Under normal temperature and pressure, according to the liquid-solid mass ratio of 1g:4ml, K 2 PtCl 4 Soluble in water, according to K 2 PtCl 4 : KI is 1g: 1.7g ratio to weigh KI, then according to the liquid-solid mass ratio of 1g: 6ml, dissolve KI in water and add the KI solution dropwise to K under the condition of dark stirring 2 PtCl 4 solution, after 30 minutes of reaction, press K 2 PtCl 4 : (1R,2R)-1,2 cyclohexanediamine is 1g:0.30g Weigh (1R,2R)-1,2 cyclohexanediamine, according to the liquid-solid mass ratio of 1g:4ml, the (1R, 2R)-1,2 cyclohexanediamine was diluted with water and added to the solution, reacted for 2 hours and filtered to obtain a yellow precipitate, first washed the filter cake with water for 4 times and the filtrate was neutral, then washed the filter cake with absolute ethanol for 3 times , and then dried at 70°C to obtain PtC with a purity ≥ 98% 6 h 14 N 2 I 2
[0031] See accompanying drawing technological process. Weigh 5.63g...
Embodiment 3
[0036] Under normal temperature and pressure, according to the liquid-solid mass ratio of 1g:5ml, K 2 PtCl 4 Soluble in water, according to K 2 PtCl 4 : KI is 1g: 1.8g ratio to weigh KI, and then according to the liquid-solid mass ratio of 1g: 8ml, dissolve KI in water and add the KI solution to K under the condition of avoiding light and stirring. 2 PtCl 4 solution, after reacting for 30min, press K 2 PtCl 4 : (1R,2R)-1,2 cyclohexanediamine is 1g:0.32g Weigh (1R,2R)-1,2 cyclohexanediamine, according to the liquid-solid mass ratio of 1g:5ml, the (1R, 2R)-1,2 cyclohexanediamine was diluted with water and added to the solution, reacted for 2 hours and filtered to obtain a yellow precipitate, first washed the filter cake with water for 5 times and the filtrate was neutral, then washed the filter cake with absolute ethanol for 4 times , and then dried at 70°C to obtain PtC with a purity ≥ 98% 6 h 14 N 2 I 2 .
[0037] See accompanying drawing process flow. Weigh 5.63g ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com