Method for synthesizing micro/nano zeolitic imidazolate frameworks (ZIFs)
A technology for zeolite imidazolate and framework structure, which is applied in the field of synthesizing zeolite imidazolate micro-nano framework structure materials, can solve problems such as unfavorable large-scale preparation, waste of organic ligands, time-consuming and energy consumption, etc., and achieves high yield and energy saving , the effect of good appearance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1, rapid preparation of ZIF-8 at room temperature
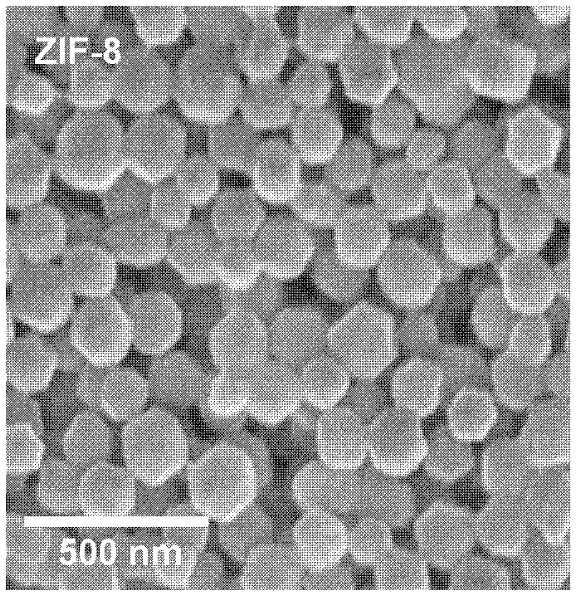
[0025] Zn(OAc) 2 2H 2 O (zinc acetate dihydrate) was dissolved in 2 mL of water to make its concentration 0.3M. Dissolve mIm (2-methylimidazole) in 2 mL of water to a concentration of 0.6M. At room temperature, 2 mL of zinc acetate (0.3 M) aqueous solution was added to the above mIm aqueous solution, and stirred for 5 minutes. The resulting precipitate was washed with ethanol and centrifuged, and the supernatant was discarded. Repeat the above operation at least three times, and finally disperse with ethanol. First, it was characterized by SEM (see figure 1 ), it can be seen that the obtained ZIF-8 material is a dodecahedron with a particle size of about 100 nm.
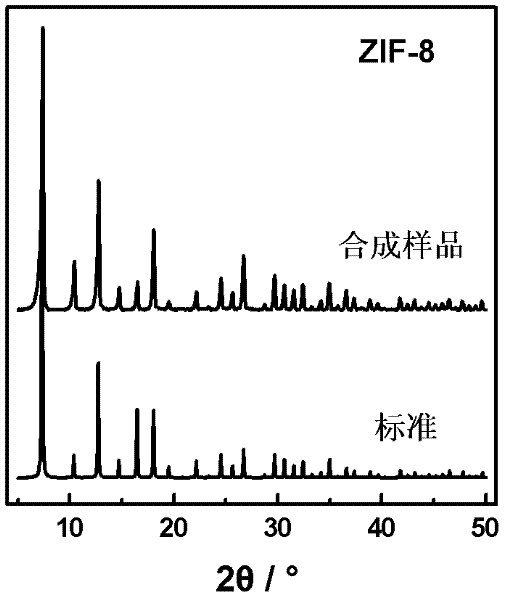
[0026] Gained ZIF-8 nanomaterials were characterized by XRD, such as figure 2 shown. As can be seen, the obtained ZIF-8 nanometer material and standard XRD spectrogram (K.Park, Z.Ni, A. , J.Choi, R.Huang, F.Uribe-Romo, H.Chae, M.O'Keeffe, O...
Embodiment 2
[0027] Example 2, rapid preparation of ZIF-61 at room temperature
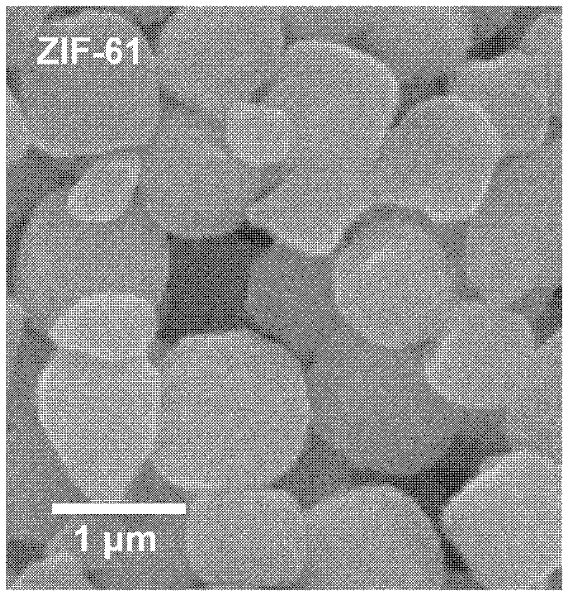
[0028] Zn(OAc) 2 2H 2 O (zinc acetate dihydrate) was dissolved in 2 mL of water to make its concentration 0.3 M; Im (imidazole, 1.8 M) and mIm (2-methylimidazole, 0.6 M) were mixed and dissolved in 2 mL of water. At room temperature, 2 mL of zinc acetate (0.3 M) aqueous solution was added to the above mixed aqueous solution of Im and mIm, and stirred for 5 minutes. The resulting precipitate was washed with ethanol and centrifuged, and the supernatant was discarded. Repeat the above operation at least three times, and finally disperse with ethanol. Then it was characterized by SEM (see image 3 ), it can be seen that the obtained material is ZIF-61 with a particle size of about 1 μm.
[0029] The obtained ZIF-61 micron material is characterized by powder XRD, as Figure 4 shown. As can be seen, the obtained ZIF-61 micron material and standard XRD spectrum (R.Banerjee, A.Phan, B.Wang, C.Knobler, H.Furukaw...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com