Mangiferin aglycone crystal forms, and composition, preparation method and application thereof
A technology of aglycone crystals and mangoes, applied in the field of medicinal chemistry, can solve problems affecting the quality, safety, effectiveness and application of drugs, affecting the processing performance of preparations, and different microscopic crystal structures.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Example 1: Preparation of Form I (Form I was prepared with 70% ethanol without adding seed crystals)
[0047] Add 500mL of 70% ethanol and 150g of water to 5.5g of mangiferin aglycone, heat and reflux for 2 hours, stop heating, let it cool down to 50°C naturally, control the temperature at 45±5°C for 2 hours, the product begins to have a small amount of crystallization, The temperature was lowered to 10±5° C. at a cooling rate of 10-20° C. per hour, and the crystallization was continued for 8 hours. The crystals were filtered out, washed with a small amount of absolute ethanol, and vacuum-dried at 70±5° C. to obtain 5.1 g of mangiferin aglycone crystal form I, with a yield of 93%.
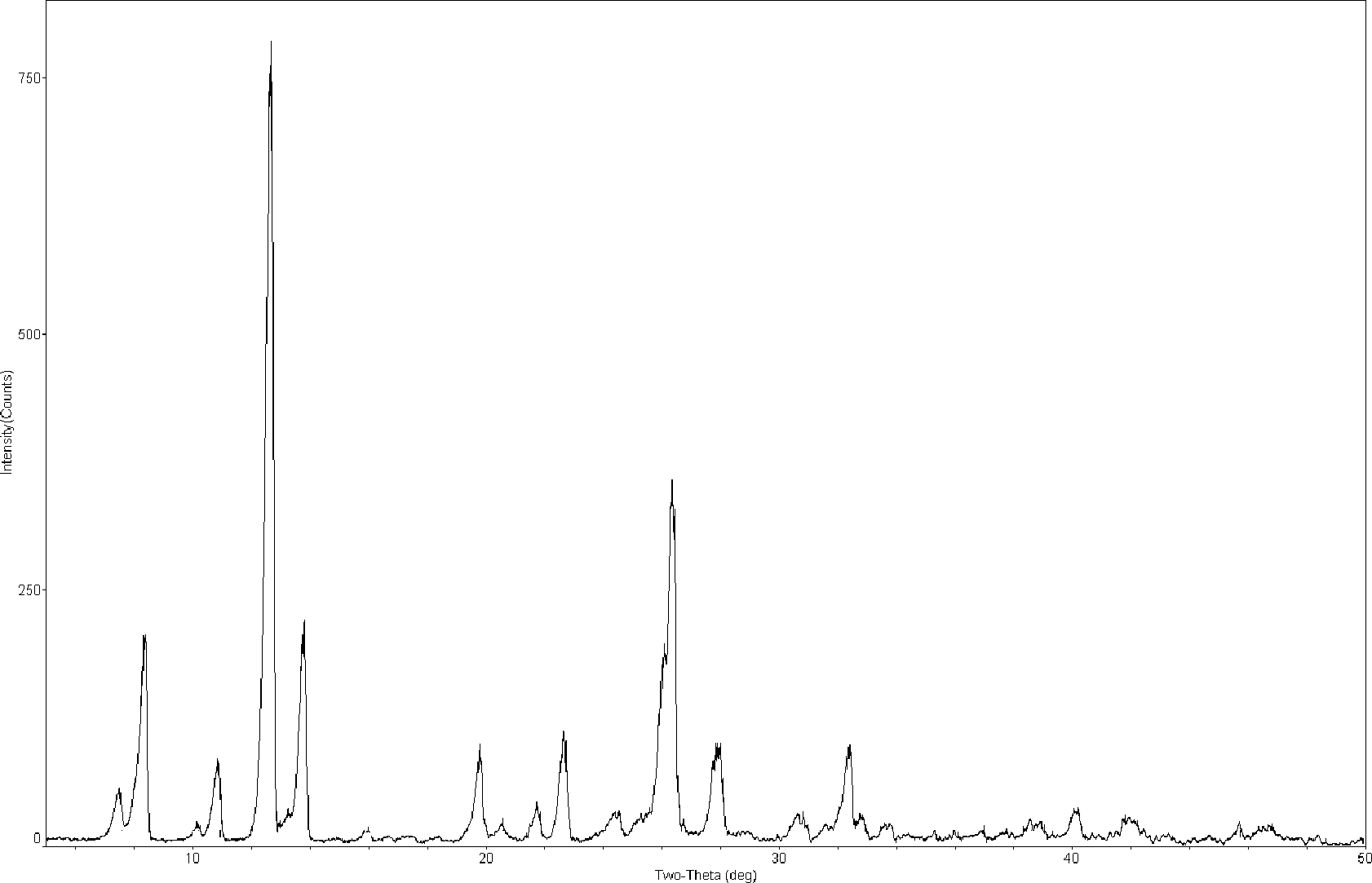
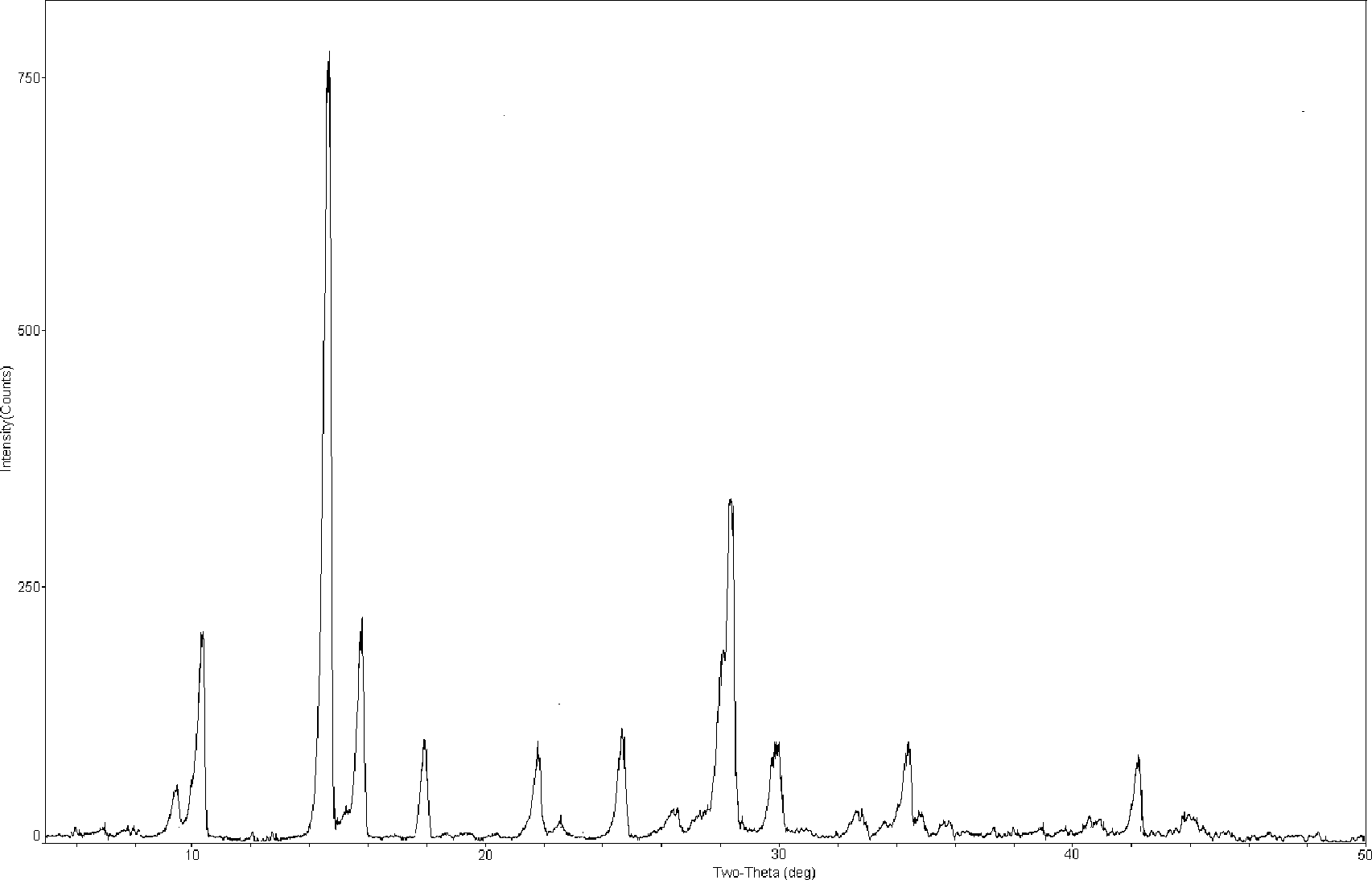
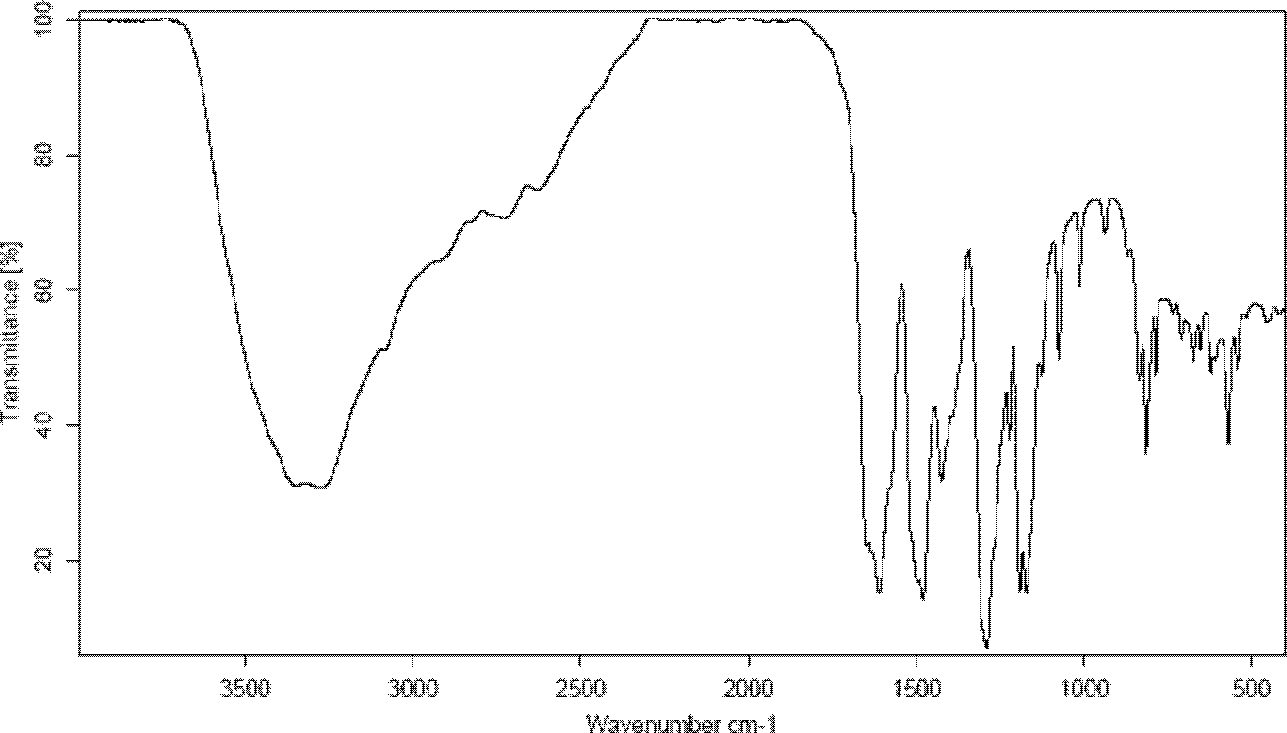
[0048] Carry out X-ray powder diffraction and infrared spectrum detection to the obtained mangiferin aglycone crystal form I, the results are shown in figure 1 and image 3 . Infrared spectrum of mangiferin aglycon form I at 3346, 3272, 1609, 1478, 1423, 1288, 1189, 1170, 1075, 812 and 56...
Embodiment 2
[0051] Example 2: Preparation of Form I (Form I was prepared with absolute ethanol without adding seed crystals)
[0052] Add 500mL of absolute ethanol to 10g of mangiferin aglycone, heat to reflux to dissolve, stop heating, let it cool down to 45°C naturally, control the temperature at 45±5°C for 2 hours, and then drop it by 10-20°C per hour The cooling rate brought the temperature down to 10±5°C, at which temperature crystallization was continued for 6 hours. The crystals were filtered out, washed with a small amount of absolute ethanol, and vacuum-dried at 70±5° C. to obtain 8.9 g of mangiferin aglycone crystal form I, with a yield of 89%. X-ray diffraction and infrared spectrum detection are consistent with the results of Example 1.
Embodiment 3
[0053] Example 3: Preparation of crystal form I (preparation of crystal form I with 90% methanol without adding seed crystals)
[0054] Add 400mL of 90% methanol to 10g of mangiferin aglycone, heat to reflux to dissolve it, stop heating, let it cool down to 30°C naturally, put it in the refrigerator and lower the temperature to 5±2°C at a cooling rate of 15-20°C per hour After 10 hours of crystallization, the crystals were filtered out, washed with a small amount of anhydrous methanol, and dried in vacuum at 60±5° C. to obtain 8.7 g of mangiferin aglycone crystal form I, with a yield of 87%. X-ray diffraction and infrared spectroscopic analysis are consistent with the results of Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com