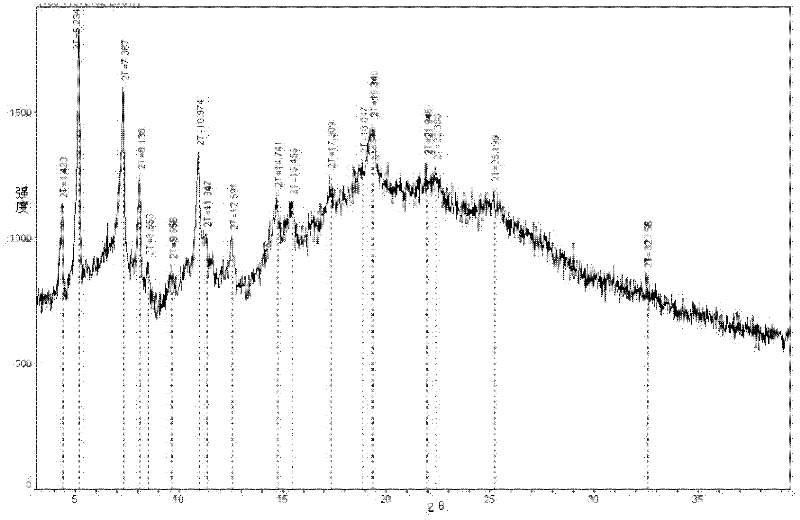
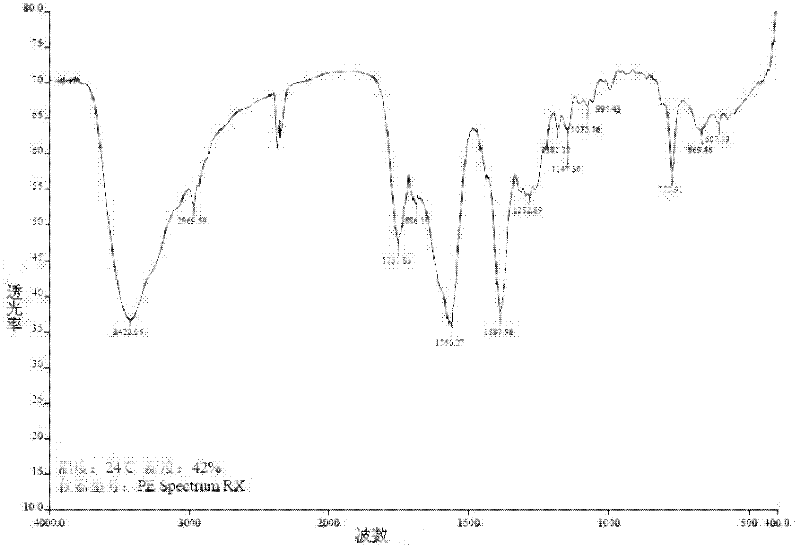
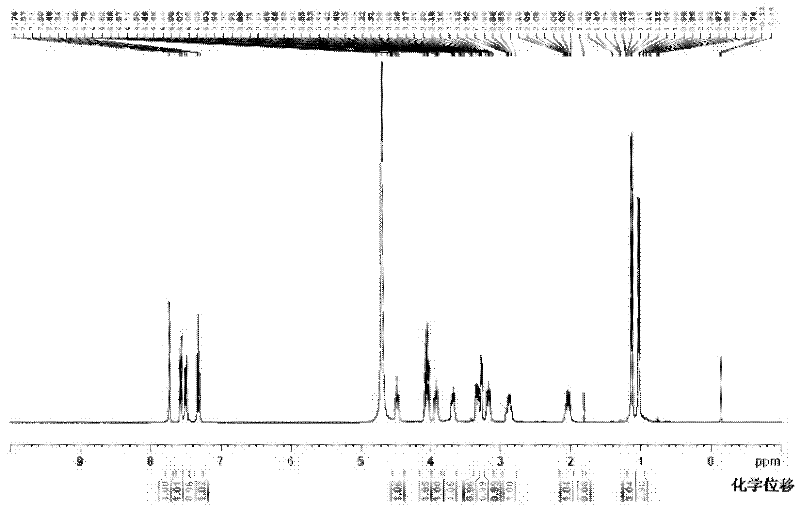
Ertapenem monosodium salt crystal and preparation method thereof
A technology of ertapenem and monosodium salt, which is applied in the field of organic chemistry, can solve the problems of poor stability of amorphous ertapenem monosodium salt and whether the unreported crystal form retains stability, etc., achieving good stability, The effect of low residual solvent amount and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] 1.86g NaHCO 3 Dissolve in 60ml of distilled water; cool to 0-5°C, add ertapenem monosodium salt raw material 10.0g, dissolve; adjust pH=5.5 with 3mol / L acetic acid-methanol solution at 0-5°C; add 2g of activated carbon Decolorize for 20 minutes, filter out activated carbon under the protection of argon and ice-water bath; add 100ml of methanol, cool to -20~-15℃, add 50ml of n-propanol dropwise, stir at a slow speed for 40min, a large amount of solids are precipitated, then add the remaining 100ml of n-propanol, stirred and crystallized for 40min after dropping; filtered, washed the filter cake with a small amount of acetone, and drained the solvent with an oil pump to obtain 7.4g of white solid; finally passed a flow of aqueous argon with a dew point of 4-6°C for 24h to obtain the Ertapenem monosodium salt crystal 7.2g.
Embodiment 2
[0058] Dissolve 0.88g NaOH in 120ml distilled water; cool to 0-5°C, add ertapenem monosodium salt raw material 10.0g, dissolve; adjust pH=5.5 with 3mol / L acetic acid-methanol solution at 0-5°C Add 2g of activated carbon for decolorization for 20 minutes, filter the activated carbon under argon protection and ice-water bath; add 100ml of methanol, cool to -20~-15°C, add 0.1g of seed crystals, and dropwise add 150ml of n-propanol, stir and analyze crystallize for 40 min; filter, wash the filter cake with a small amount of acetone, and drain the solvent with an oil pump to obtain 7.2 g of white solid; finally pass a flow of aqueous argon with a dew point of 0-4°C for 24 h to obtain the ertapenem monosodium salt crystal 7.0 g (recorded as batch 1).
[0059] The above steps were repeated two more times to obtain 7.4 g (referred to as batch 2) and 7.1 g (referred to as batch 3) of ertapenem monosodium salt crystals, respectively.
[0060] Table 1 shows the solvent residues of the 3...
Embodiment 3
[0069] 4.4g Na 2 CO 3 Dissolve in 60ml of distilled water to prepare sodium-containing inorganic alkali aqueous solution; cool to 0-5°C, add 10.0g of ertapenem monosodium salt raw material, dissolve; use 3mol / L formic acid-methanol solution at 0-5°C Adjust the pH to 5.6; add 2g of activated carbon for decolorization for 20 minutes, filter out the activated carbon under argon protection and ice-water bath; add 180ml of methanol, cool to -10~-5°C, add 0.1g of seed crystals, add dropwise 180ml of n-propanol, drop After that, stir and crystallize at a slow speed for 40 minutes; filter, wash the filter cake with a small amount of ethanol, and drain the solvent with an oil pump to obtain 6.9 g of white solid; finally, pass it through an aqueous argon flow with a dew point of 10-14°C for 6 hours to obtain the Ertapene Nandan sodium salt crystal 6.8g (recorded as batch 4).
[0070] The above steps were repeated two more times to obtain 7.0 g (referred to as batch 5) and 7.1 g (refer...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com