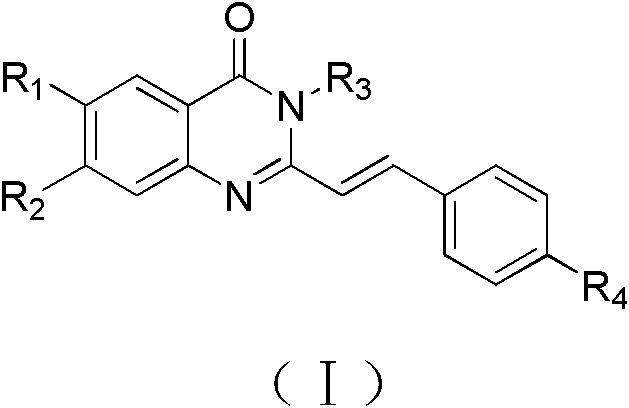
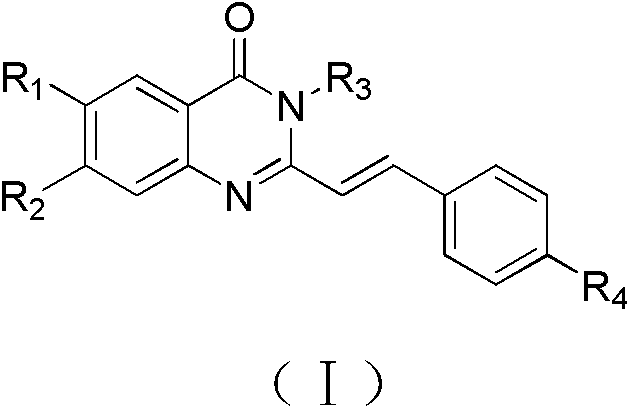
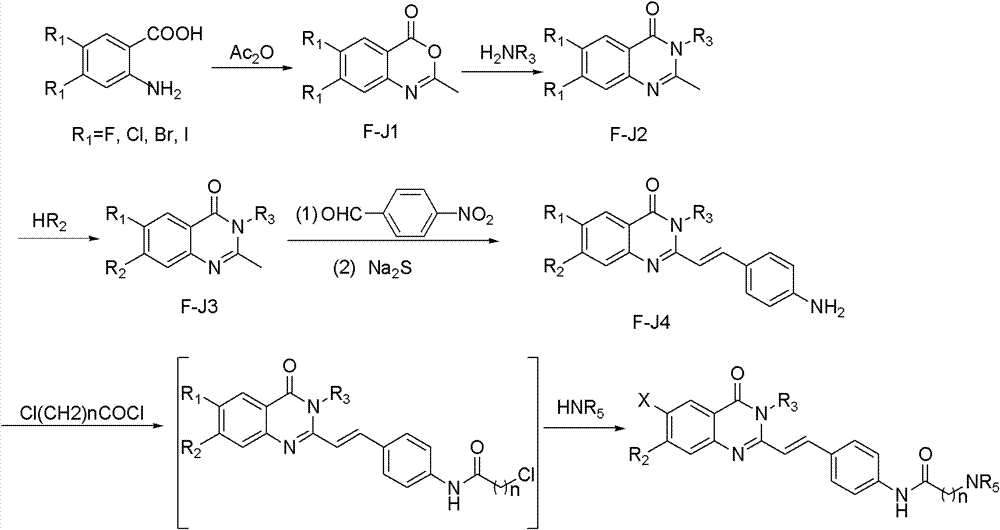
Quinnazolidone derivative, preparation method for same and application thereof
A technology of derivatives and quinazolones, which is applied in the field of quinazolones derivatives and their preparation, can solve the problems of less development and research of quinazolones, and achieve the effects of low normal cell toxicity, significant inhibitory effect, and rich research
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1: the synthesis of compound F-J11
[0042] Dissolve 0.1 mol of dry 4-nitro-2-aminobenzoic acid in 50 ml of acetic anhydride, and reflux at 130° C. for 2 hours. The reaction was cooled to room temperature and filtered with suction to obtain a white solid. The crude product was recrystallized from ethyl acetate to obtain white solid powder F-J11.
[0043] Yield: 61%; 1 H NMR (400MHz, CDCl 3 )δ7.89 (dd, J=9.2, 8.3Hz, 1H), 7.28 (dd, J=10.3, 6.9Hz, 1H), 2.40(s, 3H). LC-MS m / z: 198 [M+H ] + .
[0044]
[0045] Compound F-J11
Embodiment 2
[0046] Embodiment 2: the synthesis of compound F-J21
[0047]Dissolve 6 g of dry F-J11 (0.03 mol) in 19.5 g of aniline, stir at room temperature for 30 minutes, let stand, and filter with suction to obtain a white solid. The white solid was dissolved in 2% NaOH aqueous solution, and after 15 minutes, the clarified solution precipitated a solid, which was filtered by suction to obtain white solid powder F-J21.
[0048] Yield: 62%; 1 H NMR (400MHz, DMSO) δ8.05-7.98(m, 1H), 7.77(dd, J=11.4, 7.3Hz, 1H), 7.55(dq, J=14.2, 7.1Hz, 3H), 7.46(d, J=7.1Hz, 2H), 2.12(s, 3H).LC-MS m / z: 273[M+H] + .
[0049]
[0050] Compound F-J21
Embodiment 3
[0051] Embodiment 3: the synthesis of compound F-J31
[0052] Dissolve 5.7g of dry F-J21 (21mmol) and 10.5g of N-methylpiperazine (0.105mol) in 10ml of DMF, add a catalytic amount of sodium carbonate, react at 140°C for 2 hours, and remove by rotary evaporation under reduced pressure part of DMF, then pour the solution into 20ml of water, filter with suction to obtain a white solid, wash with water several times, and dry to obtain white solid F-J31.
[0053] Yield: 94%; 1 H NMR (400MHz, CDCl 3 )δ7.81(d, J=12.9Hz, 1H), 7.54(dt, J=14.0, 7.0Hz, 3H), 7.25(d, J=7.0Hz, 2H), 7.11(d, J=7.8Hz, 1H), 3.37-3.28(m, 4H), 2.71-2.60(m, 4H), 2.39(s, 3H), 2.21(s, 3H).LC-MS m / z: 353[M+H] + .
[0054]
[0055] Compound F-J31
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com