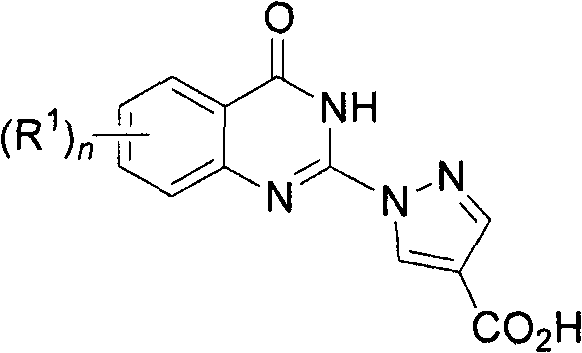
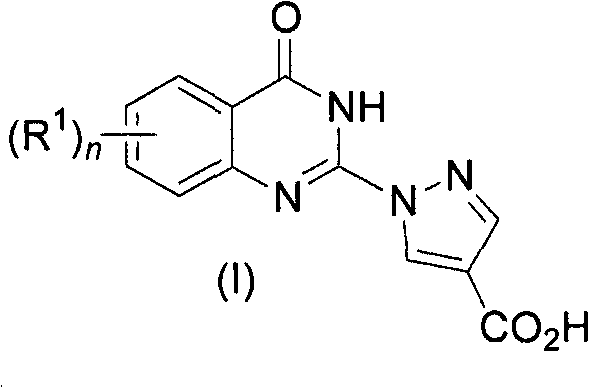
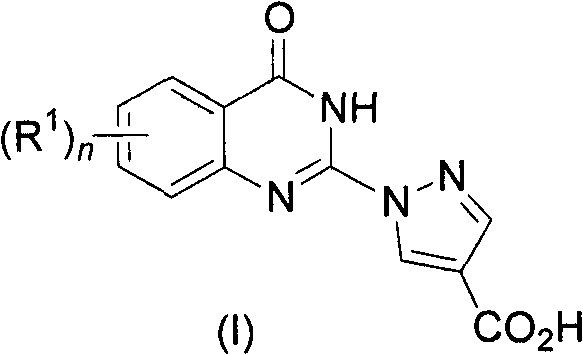
Quinazolinones as prolyl hydroxylase inhibitors
A quinazoline and pyrazole technology, applied in the field of quinazolinone compounds, can solve the problems of increased content and increased cell repopulation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0150] Chemical analysis :
[0151] In obtaining the compounds and corresponding analytical data described in the Examples below, the following experimental and analytical protocols were followed unless otherwise indicated.
[0152] The reaction mixture was magnetically stirred at room temperature (rt) unless otherwise indicated. In cases where the solutions are "dried", they are usually 2 SO 4 or MgSO 4 Such as desiccant for drying. Where mixtures, solutions and extracts are "concentrated", they are usually concentrated under reduced pressure on a rotary evaporator.
[0153] Thin layer chromatography (TLC) was performed with Merck silica gel 60F 254 2.5cm×7.5cm 250μm or 5.0cm×10.0cm 250μm pre-coated silica gel plate. Preparative thin-layer chromatography was performed on EM Science silica gel 60 F 254 This was done on a 20cm x 20cm 0.5mm precoated panel with a 20cm x 4cm concentration zone.
[0154] Normal phase flash column chromatography (FCC) was performed on s...
example 1
[0159] Example 1: 1-(7-Chloro-4-oxo-3,4-dihydro-quinazolin-2-yl)-1H-pyrazole-4-carboxylic acid .
[0160]
[0161] Step A: Preparation of 7-Chloro-1H-quinazoline-2,4-dione. A mixture of 2-amino-4-chlorobenzoic acid (2.00 g, 11.6 mmol) and urea (2.80 g, 46.6 mmol) was heated to 200°C for 1 hour. The mixture was allowed to cool to room temperature and the resulting dough was thoroughly ground with water. The product was collected by filtration (2.30 g, 100%). MS and NMR data are consistent with those that have been described previously: Organic Process Research & Development, 2003, 7, 700-706. 1 H NMR (600MHz, DMSO-d 6 ): 12.00 (br s, 2H), 8.59-8.53 (m, 1H), 7.93-7.80 (m, 2H).
[0162] Step B: Preparation of 2,4,7-trichloroquinazoline. A mixture of 7-chloro-1H-quinazoline-2,4-dione (2.0 g, 10 mmol) was suspended in ACN (50 mL), then POCl was added 3 (5.0 mL, 55 mmol). Then DIEA (5.0 mL, 28 mmol) was added. The resulting mixture was heated to reflux for 36 hours, the...
example 2
[0168] Example 2: 1-(7-Trifluoromethyl-4-oxo-3,4-dihydro-quinazolin-2-yl)-1H-pyrazole-4- carboxylic acid .
[0169]
[0170] The title compound was prepared according to the method described in Example 1 using 2-amino-4-trifluoromethylbenzoic acid in Step A. MS (CI): C 13 h 7 f 3 N 4 o 3 The mass calculated value is 324.2; m / z measured value is 323.0 [M-H] - . 1 H NMR (400MHz, DMSO-d 6 ): 14.17-12.12 (br m, 2H), 8.98 (d, J = 0.6Hz, 1H), 8.32 (d, J = 8.3Hz, 1H), 8.29 (s, 1H), 8.02 (s, 1H), 7.80 (d, J=8.3Hz, 1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com