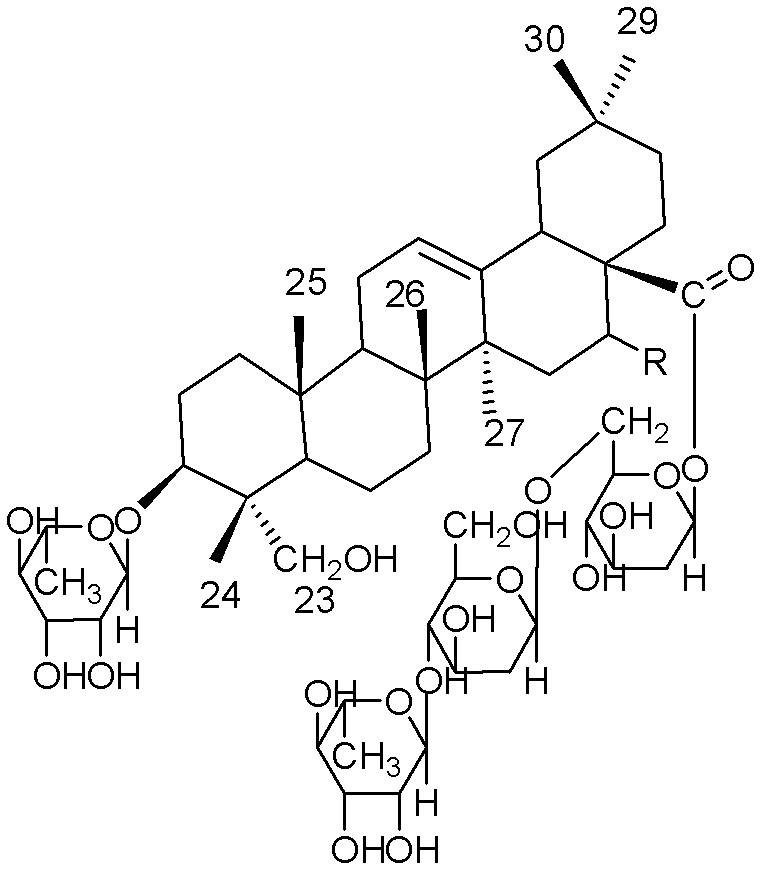
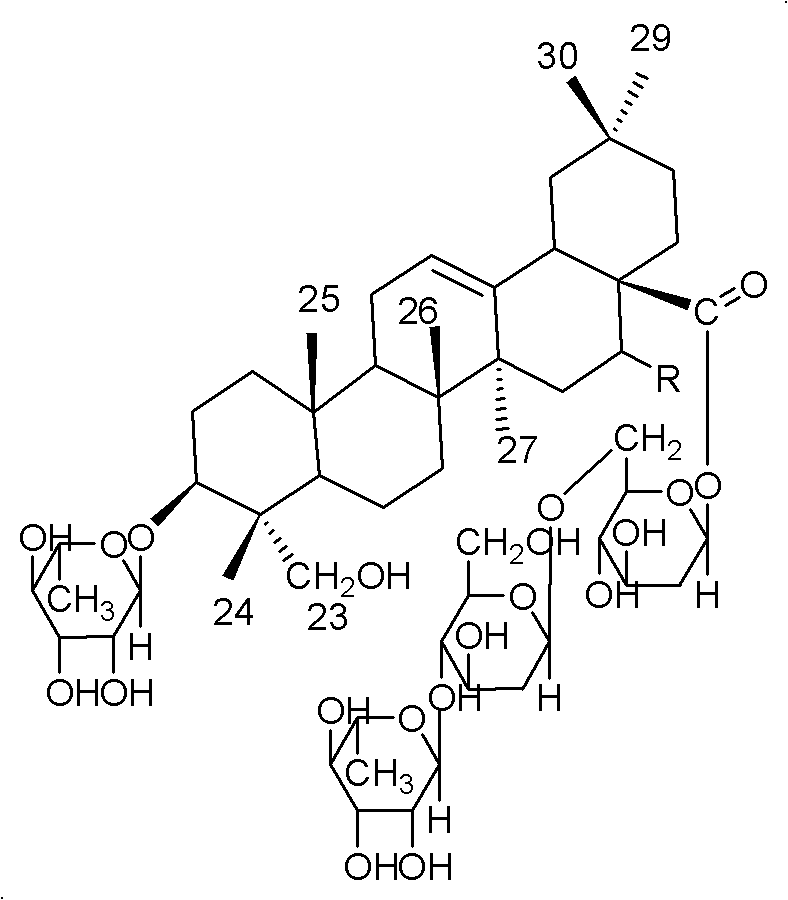
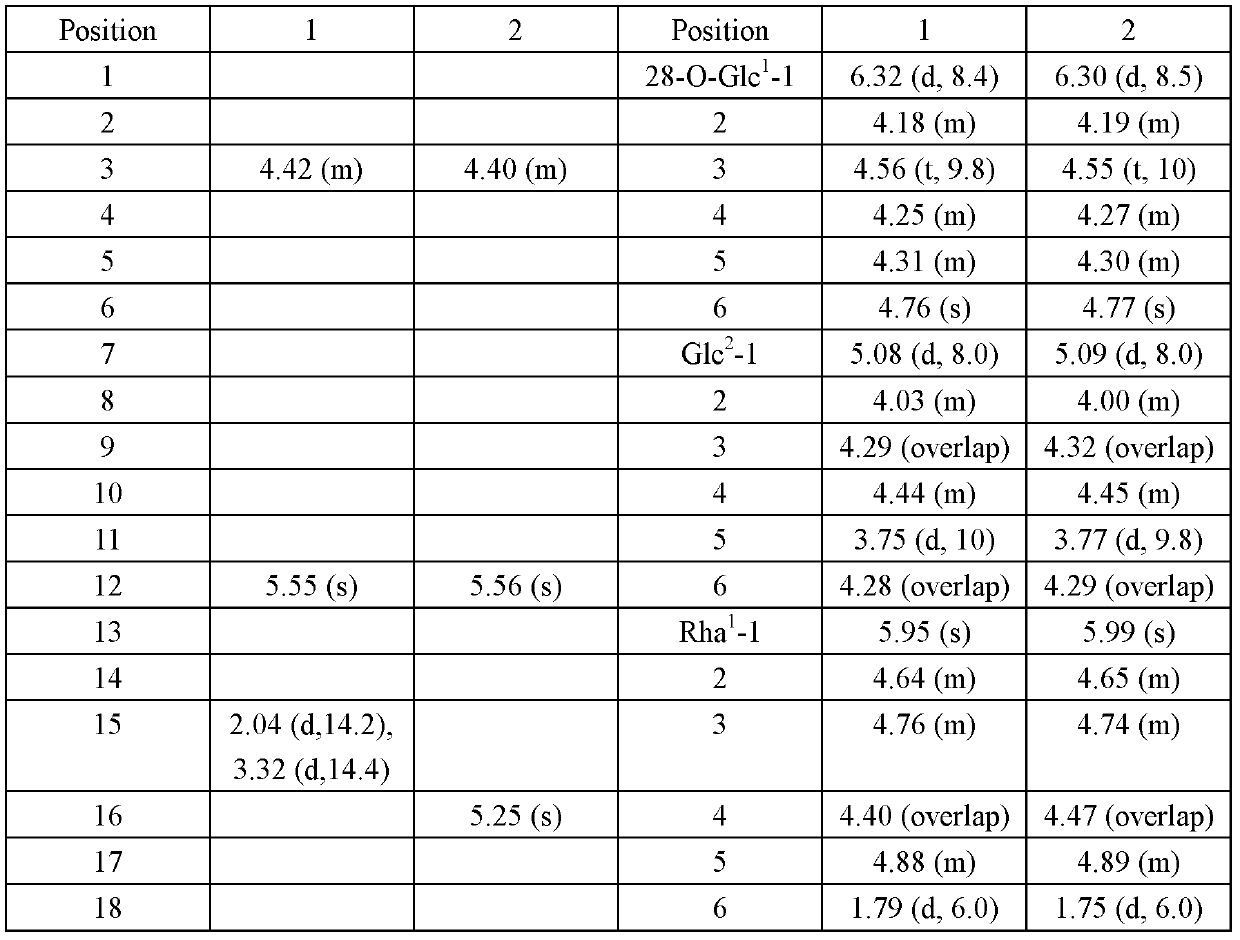
Oleanane saponin compounds and purpose thereof
A technology for oleanane and compound, which is applied in the field of new oleanane saponins compounds, can solve unseen problems and the like, and achieve the effects of preventing and treating liver diseases, simple preparation method and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Example 1. Preparation of Compound Aescin A and Aescin B
[0017] 1. Preparation of Extract Extract
[0018] Take 20kg of safflower horse chestnut medicinal material, extract by percolation with 75% ethanol aqueous solution for 6 times as usual, combine the percolation liquid, concentrate under reduced pressure until it has no alcohol smell, and extract the obtained suspension with petroleum ether and dichloromethane successively. 3 times, the petroleum ether extract and the dichloromethane extract were combined respectively, and the dichloromethane extract was concentrated under reduced pressure to obtain 200 g of the extract extract;
[0019] 2. Separation and purification
[0020] 200 g of the above extract was subjected to repeated silica gel column (100 mesh silica gel) chromatography, and gradient elution was carried out with chloroform:methanol=30:1~1:1 as the eluent, and the collected concentrations were 30:1; 20:1 1; 10:1; 1:1 part of the eluate, the fourth p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com