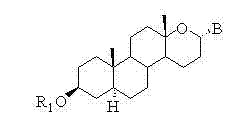
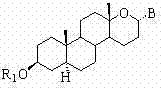
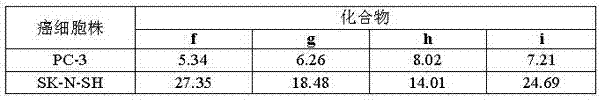
Steroid n-glycoside analogue taking dihydro-pyranoid ring as D ring and preparation and application thereof
A technology of dihydropyran ring and steroidal nitrogen glycosides, which is applied in the field of organic chemistry and achieves the effect of simple preparation method and mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Preparation of the general formula R 1 is methylsulfonyl, R 2 is a chlorine atom, R 3 Derivatives when it is a hydrogen atom, that is, compound f (3β-sulfonyl ester group-17α-(6′-chloropurine-9′-)-D-homocyclic-17a-oxa-5α-androster) preparation.
[0035] Step 1: Synthesis of compound b (3β-hydroxy-D-homocyclo-17a-oxa-5α-androst-17-one)
[0036] Take compound a (5g, 17.2mmol) in a 500mL flask, add dichloromethane (50mL) and stir to dissolve, then add methanol (250mL) and distilled water (60mL), add MMPP (8.5g, 17.2mmol) to the reaction system under stirring at room temperature ), then add the same amount of MMPP every 24h, react for 4d, and stop the reaction after the thin-layer chromatography detects that the reaction is complete; ) extraction, the organic phases were combined and washed with 10% sodium hydroxide (10mL×3) until the pH of the aqueous phase was alkaline, then washed with saturated brine (20mL×3), anhydrous Na 2 SO 4 Dry, and finally concentrate under...
Embodiment 2
[0050] Example 2 prepares R shown in the general formula 1 is methylsulfonyl, R 2 is a chlorine atom, R 3 When it is a chlorine atom, the compound g(3β-sulfonyl ester group-17α-(2′,6′-dichloropurinyl-9′-)-D-homocycle-17a-oxa-5α- androster) preparation
[0051] Add 211mg of trifluoroacetic acid to the ethyl acetate solution (50mL) of compound e (1g, 2.7mmol), then add the base 2,6-dichloropurine, heat up and reflux for 24h, lower the temperature of the system to room temperature, and depressurize The organic solvent was distilled off to obtain a white solid, and dichloromethane was added to dissolve the white solid, and the organic phase was washed with 5% NaOH solution, anhydrous Na 2 SO 4 The organic phase was dried, and the organic solvent was distilled off under reduced pressure to obtain a white solid, which was recrystallized from ethanol to obtain a white needle-like solid, that is, compound g (3β-sulfonyl ester-17α-(2′,6′-dichloropurinyl-9 '-)-D-homocyclo-17a-oxa-5...
Embodiment 3
[0053] Example 3 prepares R shown in the general formula 1 is methylsulfonyl, R 2 is a chlorine atom, R 3 When it is a derivative of a fluorine atom, the compound h(3β-sulfonyl ester group-17α-(2′-fluoro-6′-chloropurinyl-9′-)-D-homocycle-17a-oxa-5α - androster) preparation
[0054] Add 211mg of trifluoroacetic acid to the ethyl acetate solution (50mL) of compound e (1g, 2.7mmol), then add the base 2-fluoro-6-chloropurine (1.0g, 5.4mmol), heat and reflux for 24h, and The temperature of the system was lowered to room temperature, and the organic solvent was distilled off under reduced pressure to obtain a white solid. Add dichloromethane to dissolve the white solid, wash the organic phase with 5% NaOH solution, anhydrous Na 2 SO 4 The organic phase was dried, and the organic solvent was distilled off under reduced pressure to obtain a white solid, which was recrystallized from ethanol to obtain a white needle-like solid, namely compound h (3β-sulfonyl ester group-17α-(2′-fl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com