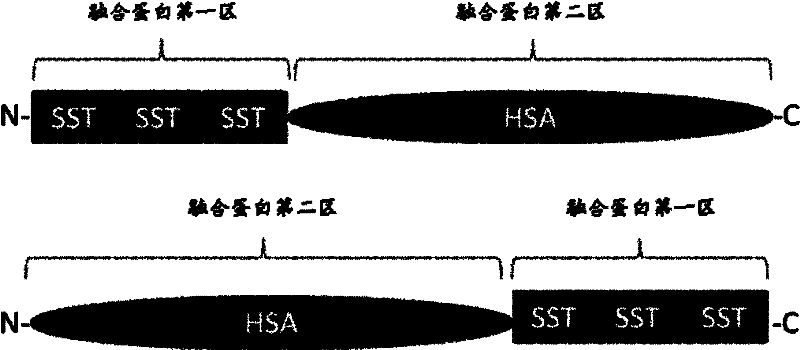
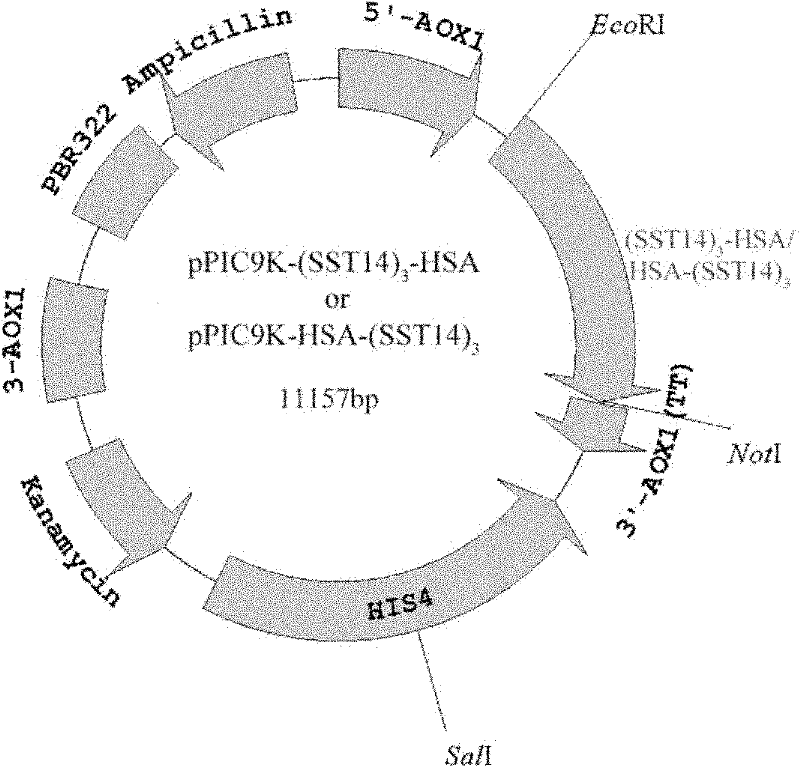
Fusion protein of human somatostatin tetradecapeptide and human serum albumin, and coding gene and preparation method thereof
A human serum albumin and fusion protein technology, applied in the field of long-acting fusion protein drugs, can solve the problem that the physiological function is inferior to natural SST, etc., and achieve the effects of changing the pharmacokinetic characteristics, reducing the mortality rate and prolonging the retention time.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0063] 1. Cloning of the SST-14 triplet gene
[0064] a. (SST-14) 3 Oligonucleotide annealing
[0065] Artificially synthesized two complementary (SST-14) 3 Oligonucleotides (see (SS-14) 3 - oligonucleotide sequence 1 and (SS-14) 3 - oligonucleotide sequence 2). 10l to 70l ddH for each of oligonucleotide sequences 1 and 2 at a concentration of 100M 2 O, simultaneously add 10l 10 annealing buffer to prepare 10M (SS14) 3 - Oligonucleotide mixed solution. After heating the oligonucleotide mixed solution at 95C for 2 minutes, let the oligonucleotide mixed solution cool down to room temperature (25C-30C).
[0066] b. (SST-14) 3 extend
[0067] 30 l annealed oligonucleotide template, 10 l10 Klenow buffer, 10 l 25 mM dNTPs, mixed, incubated at 37C for 30 minutes. Add 4 l of 100 mM EDTA to stop the reaction.
[0068] c. (SST-14) 3 PCR amplification
[0069] The reaction system is: 0.5l of 10μmol / L PS1 and PS2 primers, 0.5l of 10mmol / L dNTP, 2.5μl of 10pfu buffer, 0.5...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Average weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com