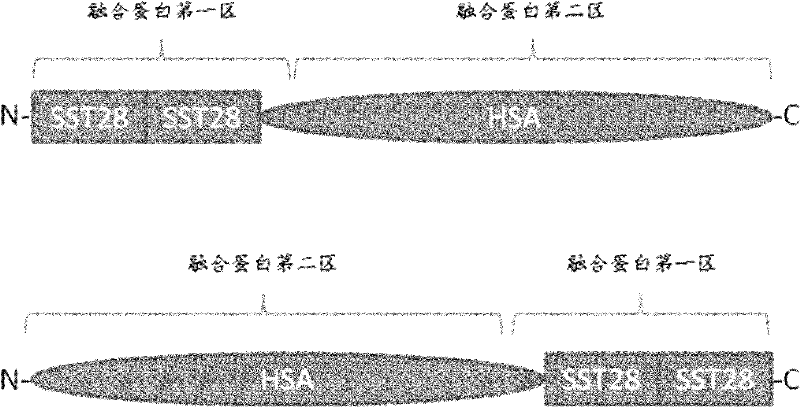
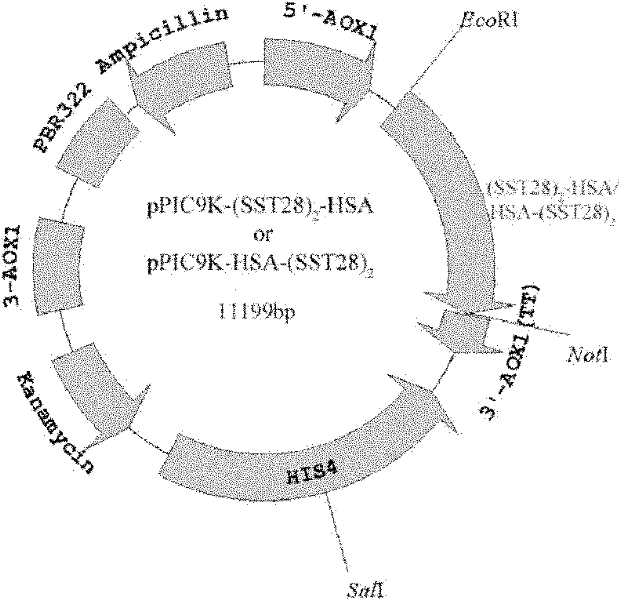
Fusion protein of human somatostatin 28 peptide and human serum albumin, encoding gene of fusion protein and preparation method for fusion protein
A human serum albumin and fusion protein technology, applied in the field of long-acting fusion protein drugs, can solve the problems of poor selectivity, limited clinical application, and physiological functions inferior to natural SST, etc., to prolong the retention time, reduce mortality, and change Effect of Pharmacokinetic Properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
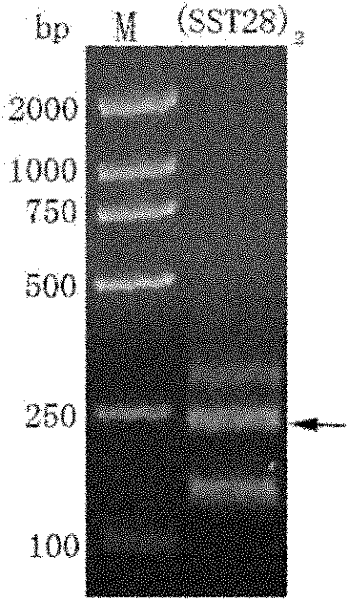
[0066] 1. Cloning of the SST-28 duplex gene
[0067] a. (SST-28) 2 Oligonucleotide annealing
[0068] Artificially synthesized two complementary (SST-28) 2 Oligonucleotides (see (SS-28) 2 - oligonucleotide sequence 1 and (SS-28) 2 - oligonucleotide sequence 2). Take 10 μl to 70 μl ddH each of oligonucleotide sequences 1 and 2 at a concentration of 100 μM 2 O, simultaneously add 10 μl 10× annealing buffer to prepare 10 μM (SS28) 2 - Oligonucleotide mixed solution. After heating the oligonucleotide mixture solution at 95°C for 2 minutes, let the oligonucleotide mixture solution cool down to room temperature (25°C-30°C) naturally.
[0069] b. (SST-28) 2 extend
[0070] 30 μl annealed oligonucleotide template, 10 μl 10× Klenow buffer, 10 μl 25 mM dNTPs, mix and incubate at 37°C for 30 minutes. Add 4 μl of 100 mM EDTA to stop the reaction.
[0071] c. (SST-28) 2 PCR amplification
[0072] The reaction system is: 0.5 μl of 10 μmol / L PS1 and PS2 pri...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com