Manufacturing process of electrolyte for oxidation reduction cell
A manufacturing method and electrolyte technology, which are applied in indirect fuel cells, final product manufacturing, sustainable manufacturing/processing, etc., can solve the problems of delaying the popularization of redox batteries, complex manufacturing processes, and dangers, and achieve automatic operation that is simple and easy to operate. Easy drug administration and easy observation of effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
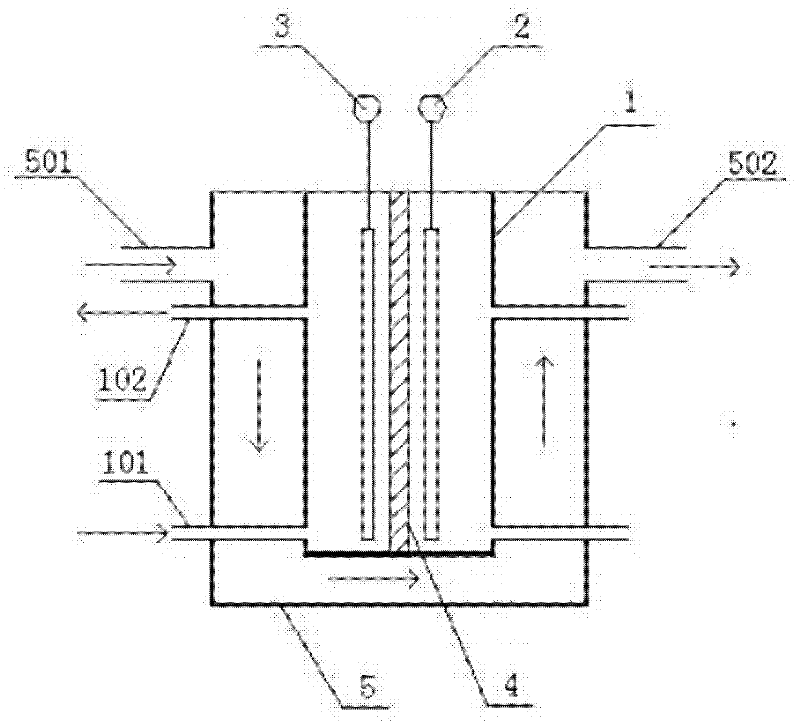
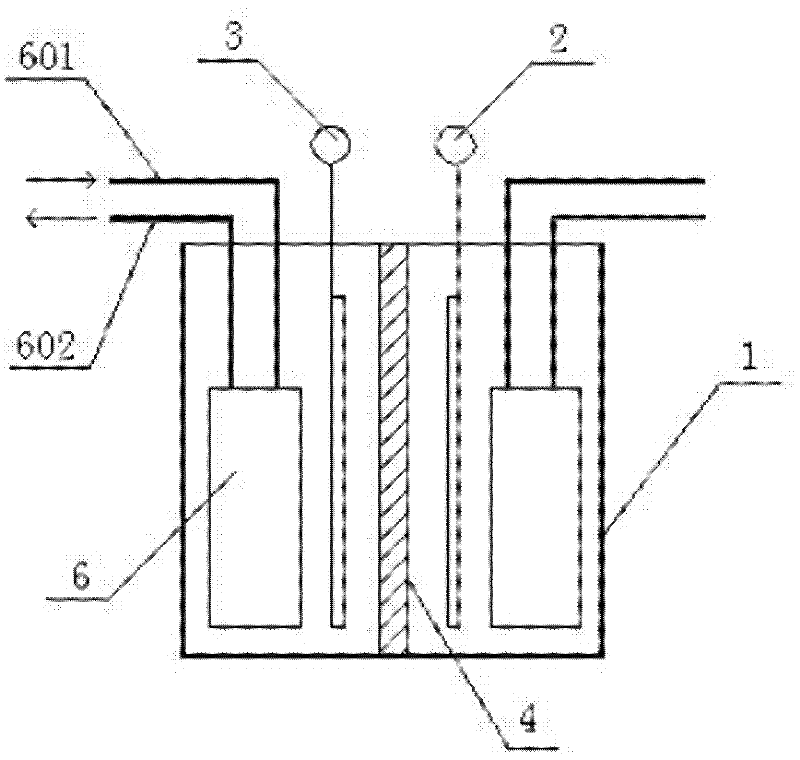
Image
Examples
Embodiment 1
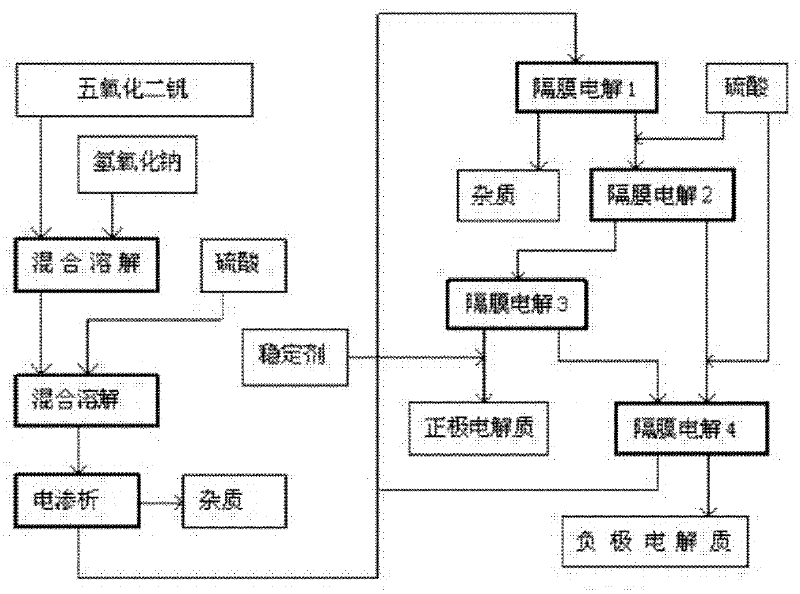
[0041] Such as figure 1 A method for manufacturing an electrolyte for a redox battery shown, the process steps are as follows:
[0042] (1) Slowly add vanadium pentoxide to the aqueous sodium hydroxide solution with a concentration of 20wt% and keep stirring, the stirring rate is controlled at 120rpm, and the addition of vanadium pentoxide is 25wt% of the quality of the alkaline aqueous solution. The speed of adding vanadium pentoxide is preferably not to produce a large amount of precipitates, and the adding time is 30 minutes;
[0043] (2) After the vanadium pentoxide addition is completed, continue to stir, and the stirring rate is controlled at 30rpm, and continues to stir for 50 minutes to make it fully dissolve;
[0044] (3) Slowly add sulfuric acid with a concentration of 25 wt% into the above system to react to generate vanadyl(IV) sulfate, stop adding when the pH value of the system reaches 2.0 to 2.2, and continue the reaction for 30 minutes;
[0045] (4) remove so...
Embodiment 2
[0052] Such as figure 1 A method for manufacturing an electrolyte for a redox battery shown, the process steps are as follows:
[0053] (1) Slowly add vanadium pentoxide to the aqueous sodium hydroxide solution with a concentration of 30wt% and keep stirring, the stirring rate is controlled at 80rpm, and the addition of vanadium pentoxide is 40wt% of the quality of the alkaline aqueous solution. The speed of adding vanadium pentoxide is advisable not to produce a large amount of precipitates, and the adding time is 60 minutes;
[0054](2) After the vanadium pentoxide addition is completed, continue to stir, and the stirring rate is controlled at 50rpm, and continues to stir for 30 minutes to make it fully dissolve;
[0055] (3) Slowly add sulfuric acid with a concentration of 20 wt% into the above system to react to generate vanadyl(IV) sulfate, stop adding when the pH value of the system reaches 2.0 to 2.2, and continue the reaction for 20 minutes;
[0056] (4) remove sodiu...
Embodiment 3
[0062] Such as figure 1 A method for manufacturing an electrolyte for a redox battery shown, the process steps are as follows:
[0063] (1) Slowly add vanadium pentoxide to the potassium hydroxide aqueous solution with a concentration of 20wt% and keep stirring, the stirring rate is controlled at 150rpm, and the addition of vanadium pentoxide is 25wt% of the potassium hydroxide aqueous solution quality. The speed of adding vanadium pentoxide is preferably not to produce a large amount of precipitates, and the adding time is 30 minutes;
[0064] (2) After the vanadium pentoxide addition is completed, continue to stir, and the stirring rate is controlled at 60rpm, and continues to stir for 30 minutes to make it fully dissolve;
[0065] (3) Slowly add sulfuric acid with a concentration of 30wt% into the above system to react to generate vanadyl(IV) sulfate, stop adding when the pH value of the system reaches 2.0 to 2.2, and continue the reaction for 30 minutes;
[0066] (4) rem...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com