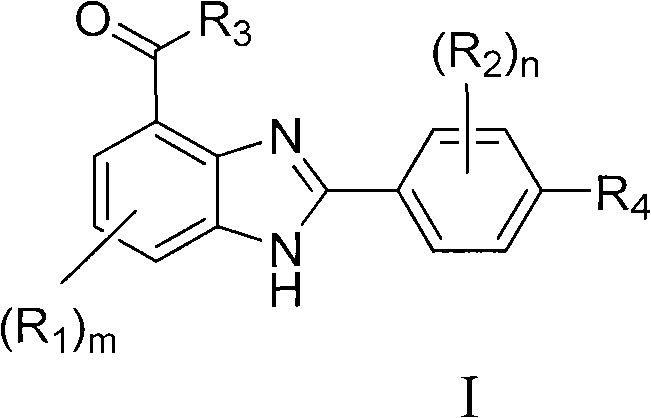
2-phenyl-1h-benzimidazole-4-formic ether derivative serving as PARP (poly(ADP-ribose)polymerase) inhibiting agent
A technology of benzimidazole and methyl formate, applied in the field of medicinal chemistry, can solve problems such as no drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
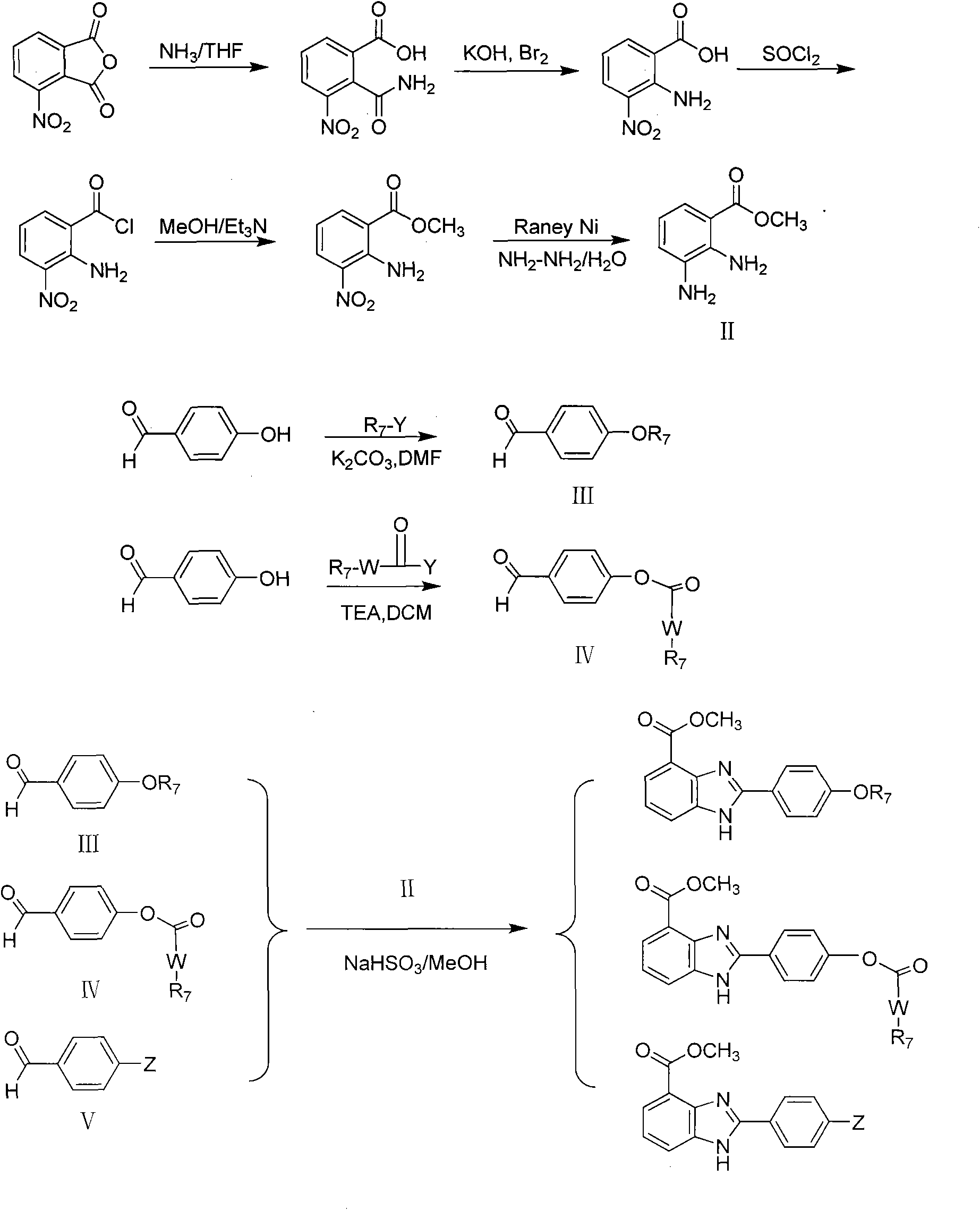
[0068] Synthesis of key intermediate 2,3-diaminobenzoic acid methyl ester (II)
[0069] (a) 3-nitro-2-carbamoylbenzoic acid
[0070] Dissolve 3-nitrophthalic anhydride (1.0g, 5.2mmol) in 20ml of dry tetrahydrofuran, stir at room temperature to dissolve, slowly add an appropriate amount of ammonia water dropwise, a white solid precipitates, continue to stir at room temperature for 4h, let stand for 20min, filter A white solid was obtained, left to stand, the residual solvent was evaporated to dryness, and recrystallized with dilute hydrochloric acid (10%) to obtain 0.9 g of a white solid, yield 87%, mp 208-208.5°C. 1 H-NMR (DMSO-d 6 )d: 7.56 (1H, brs, -NH 2 ), 7.68-7.73 (1H, t, Ar-H), 7.79 (1H, brs, -NH 2 ), 8.10-8.13 (1H, m, Ar-H), 8.16-8.19 (1H, m, Ar-H), 13.45 (1H, brs, -COOH); MS m / z: 211.0 [M+H] + , 233.0[M+Na] + , 249.0[M+K] + ; IR(KBr, cm -1 ): 3463, 3188, 1668, 1529.
[0071] (b) 2-Amino-3-nitrobenzoic acid
[0072] Dissolve 5g of KOH in 20ml of water to prepar...
Embodiment 2
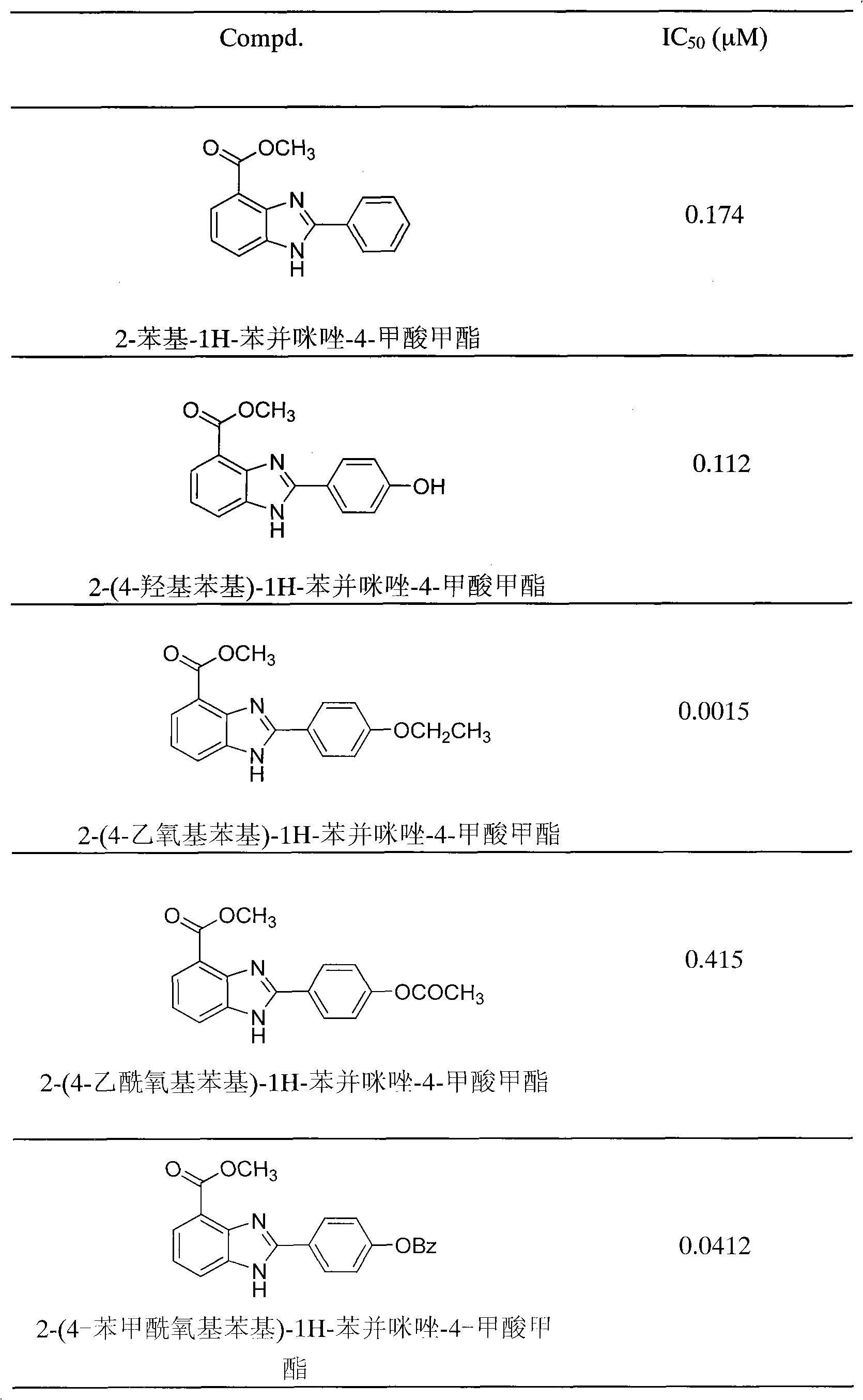
[0079] Synthesis of 2-(4-hydroxyphenyl)-1H-benzimidazole-4-carboxylic acid methyl ester
[0080] Methyl 2,3-diaminobenzoate (0.28g, 1.6mmol) and p-hydroxybenzaldehyde (0.18g, 1.5mmol) were dissolved in 20ml of methanol and an appropriate amount of NaHSO was added 3 , Heated to reflux for 6h, filtered the reaction solution, and distilled off methanol. Add appropriate amount of silica gel to make sand and apply column chromatography (petroleum ether: ethyl acetate = 1:2) to separate about 0.4 g of light yellow homologue, yield 81%, mp 257-258°C. 1 H-NMR (CDCl 3 )d: 3.98 (3H, s, -OCH 3 ), 6.93-6.95 (2H, m, Ar-H), 7.30-7.33 (1H, m, Ar-H), 7.79-7.81 (1H, m, Ar-H), 7.90-7.91 (1H, m, Ar-H) -H), 8.13-8.14 (2H, m, Ar-H); MS m / z: 269.3 [M+H] + ; IR(KBr, cm -1 ): 3417, 1695, 1440, 1280; Elementary Analysis: C 15 h 12 N 2 o 3 0.3H 2 O, calcd (C 65.8, H 4.64, N 10.23), Found (C 66.10, H 4.48, N 9.84).
Embodiment 3
[0082] Synthesis of 2-(4-bromophenyl)-1H-benzimidazole-4-carboxylic acid methyl ester
[0083] Refer to Example 2 for the method. Yield: 72%, mp 123-124°C. 1 H-NMR (CDCl 3 )d: 4.04 (3H, s, -CH 3 ), 7.26-7.37 (1H, m, Ar-H), 7.67-7.70 (2H, m, Ar-H), 7.92-7.97 (1H, m, Ar-H), 8.01-8.02 (2H, m, Ar-H -H), 8.03-8.05 (1H, m, Ar-H); MS m / z: 333.0 [M+H] + , 353.0[M+Na] + ; IR(KBr, cm -1 ): 3446, 1703, 1282, 750; Elementary Analysis: C 15 h 11 N 2 o 2 , calcd (C 54.40, H 3.34, N 8.45), Found (C 54.00, H 3.67, N 8.23).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com