Determination reagent for eliminating interference of ascorbic acid by uricase method and application method thereof
A technology of ascorbic acid and uricase, applied in the biological field, to achieve the effect of excellent interference performance, good stability, and strong anti-interference performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
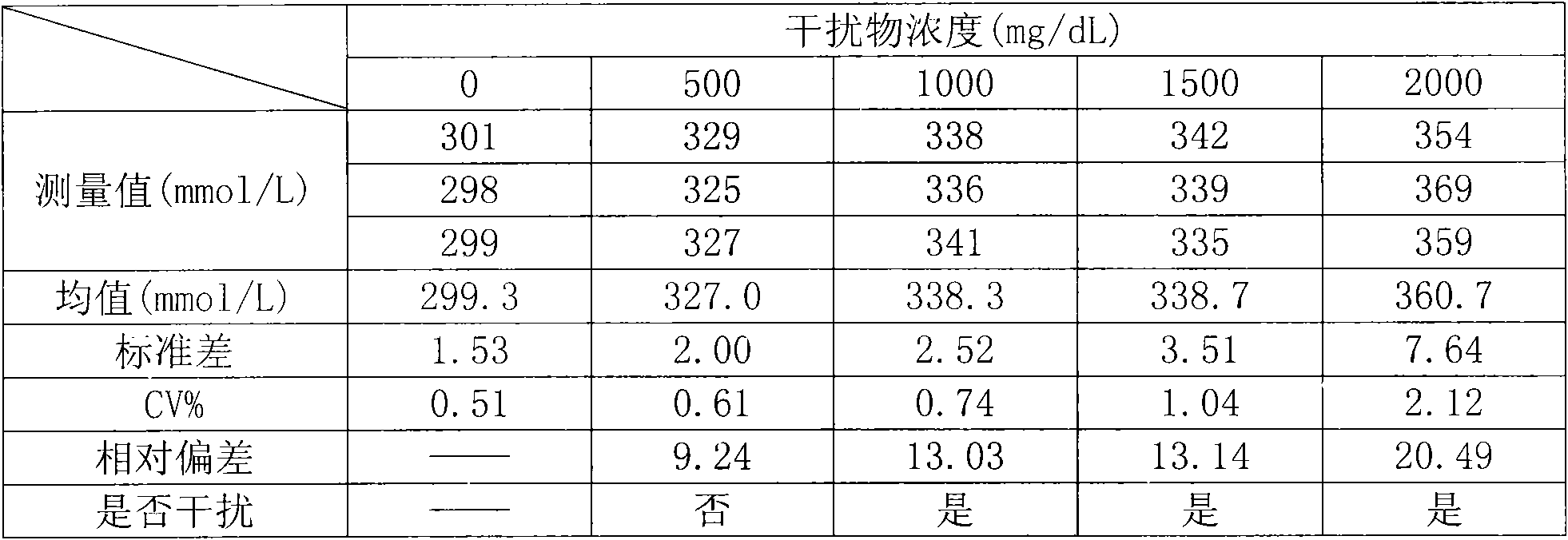
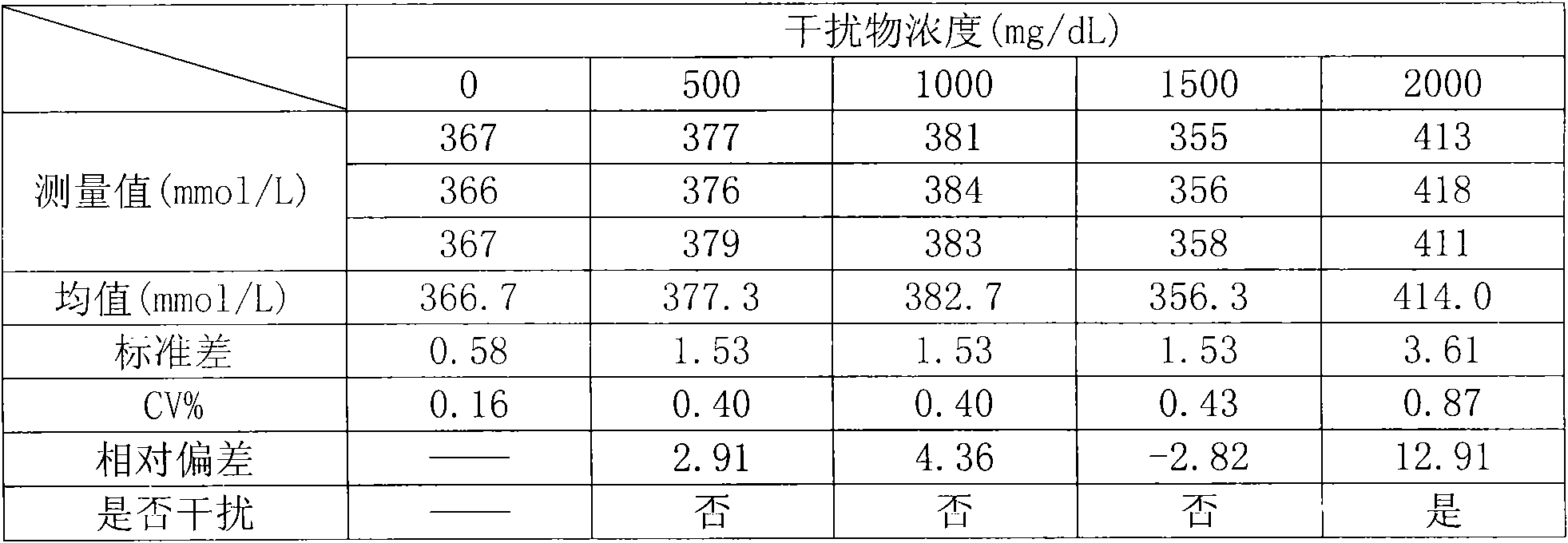
[0041] (1) Sample preparation: the sample is fasting non-hemolyzed serum or plasma (heparin anticoagulation, 0.1mg heparin can anticoagulate 1.0ml blood; EDTA anticoagulation: 1.8mg EDTA can anticoagulate 1.0ml blood). Samples should be transported and stored at low temperature. Uric acid in samples is stable for 8 hours at room temperature, 3 days at 2°C to 8°C, and 6 months at -20°C.
[0042] (2) Prepare the uric acid determination reagent according to the following ingredients and dosage:
[0043] Reagent 1:
[0044] Phosphate buffer (pH 6.0-8.0) 0.10-100g / L,
[0045] Peroxidase 0.10-10KU / L,
[0046] N-ethyl-N-(3-toluene)-N-succinylethylenediamide EMSE 0.10-10g / L,
[0047] Anti-interference enzyme 0.10-10KU / L,
[0048] Stabilizer 0.10-100g / L;
[0049] Reagent 2:
[0050] Phosphate buffer (pH 6.0-8.0) 0.10-100g / L,
[0051] Peroxidase 0.10-10KU / L,
[0052] Uricase 0.10-10KU / L,
[0053] 4-Aminoantipyrine 4-AAP 0.10-10g / L,
[0054] Stabilizer 0.10-100g / L.
[0055]The...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com