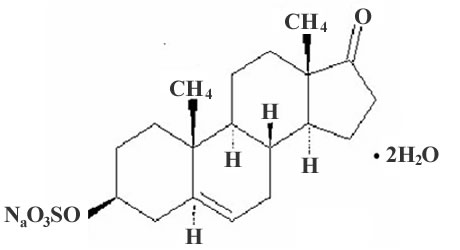
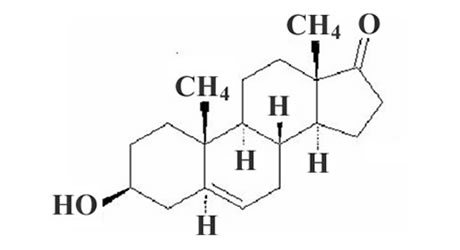
Pharmaceutical composition prepared with micronized prasterone or sodium prasterone sulfate and use thereof
A technology of prasterone sulfate sodium and prasterone, applied in the direction of drug combinations, pharmaceutical formulas, medical preparations containing active ingredients, etc., can solve the problem of no similar patents found for DHEA or DHEAS compositions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach 1
[0027] Embodiment 1, preparation of DHEA and DHEAS powder
[0028] DHEAS raw material: Jiangsu Yangzhou Pharmaceutical Factory;
[0029] DHEA raw material: Changzhou Jiaerke Pharmaceutical Group Co., Ltd.;
[0030] Jet Pulverizer (The Jet Pulverizer Company, Inc.);
[0031] Z R S -8 Drug Dissolution Apparatus (Tianjin University Radio Factory).
[0032] 1. 1. Preparation of DHEA particles with different particle sizes
[0033] Particles with a size of 50 mesh but less than 65 mesh obtained by the conventional sieving method are ordinary powder 1 (equivalent to 230-270 microns);
[0034] Particles that pass 100 mesh but are less than 120 mesh obtained by conventional sieving are general powder 2 (equivalent to 120-150 microns);
[0035] Obtain the superfine powder with a center of 10 micron normal distribution as micropowder 3 with an ultrafine jet mill;
[0036] Obtain the ultra-fine powder with a center of 0.5 micron normal distribution with the ultra-fine jet mill as fi...
Embodiment approach 2
[0042] Embodiment 2, Dissolution test of DHEAS film-coated tablets with different particle sizes
[0043] 2.1. Drugs to be tested: DHEAS common powder and micropowder as mentioned above (1. 2. Preparation of DHEAS particles with different particle sizes).
[0044] 2.2. Prescription: Each tablet weighs 100 (mg);
[0045] DHEAS: 50;
[0046] Sodium carboxymethyl cellulose: 30;
[0047] Cornstarch: 10;
[0048] Microcrystalline Cellulose: 6;
[0049] Magnesium stearate: appropriate amount;
[0050] 5% hydroxypropyl methylcellulose: appropriate amount.
[0051] 2.3. Process: Take DHEAS, sodium carboxymethyl cellulose, corn starch, and microcrystalline cellulose to make plain tablets according to conventional processes, then use hydroxypropyl methyl cellulose as a film-forming agent, and 70% alcohol solution as a solvent. Diethyl phthalate is used as a plasticizer coating to obtain film-coated tablets.
[0052] The obtained film-coated tablets are respectively: general powde...
Embodiment approach 3
[0061] Embodiment 3, Dissolution Test of Common Powder 1, Common Powder 2, Micropowder 3, and Micropowder 4 Capsules Prepared by DHEA
[0062] 3.1. Drugs to be tested: general powder and micropowder of the above (1. 1. DHEA particles with different particle sizes).
[0063] 3.2. Prescription: Each capsule weighs 100 (mg)
[0064] DHEA: 50;
[0065] Cornstarch: 25;
[0066] Microcrystalline Cellulose: 22;
[0067] 2%HMPC: appropriate amount;
[0068] Magnesium stearate: 0.6.
[0069] 3.3. Process: Take the common powder 1, common powder 2, micro-powder III, micro-powder IV and auxiliary materials prepared by DHEA respectively, prepare granules according to the conventional process, and pack them into capsules.
[0070] Corn starch in the prescription is used as disintegrant, dispersant and diluent. Hypromellose is the binder. Magnesium stearate is a lubricant. The general powder capsule 1, the general powder capsule 2, the micro powder capsule 3 and the micro powder cap...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com