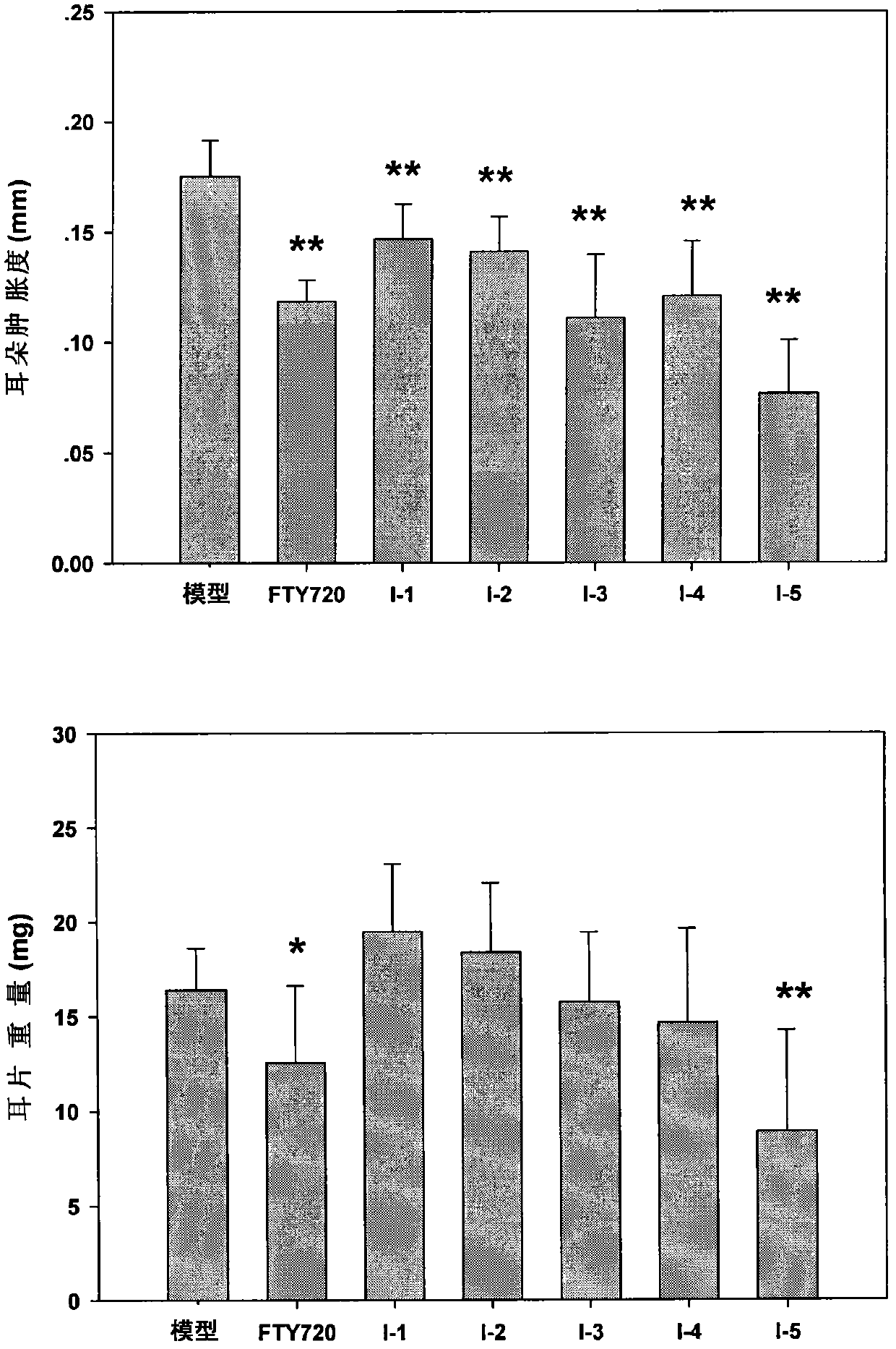
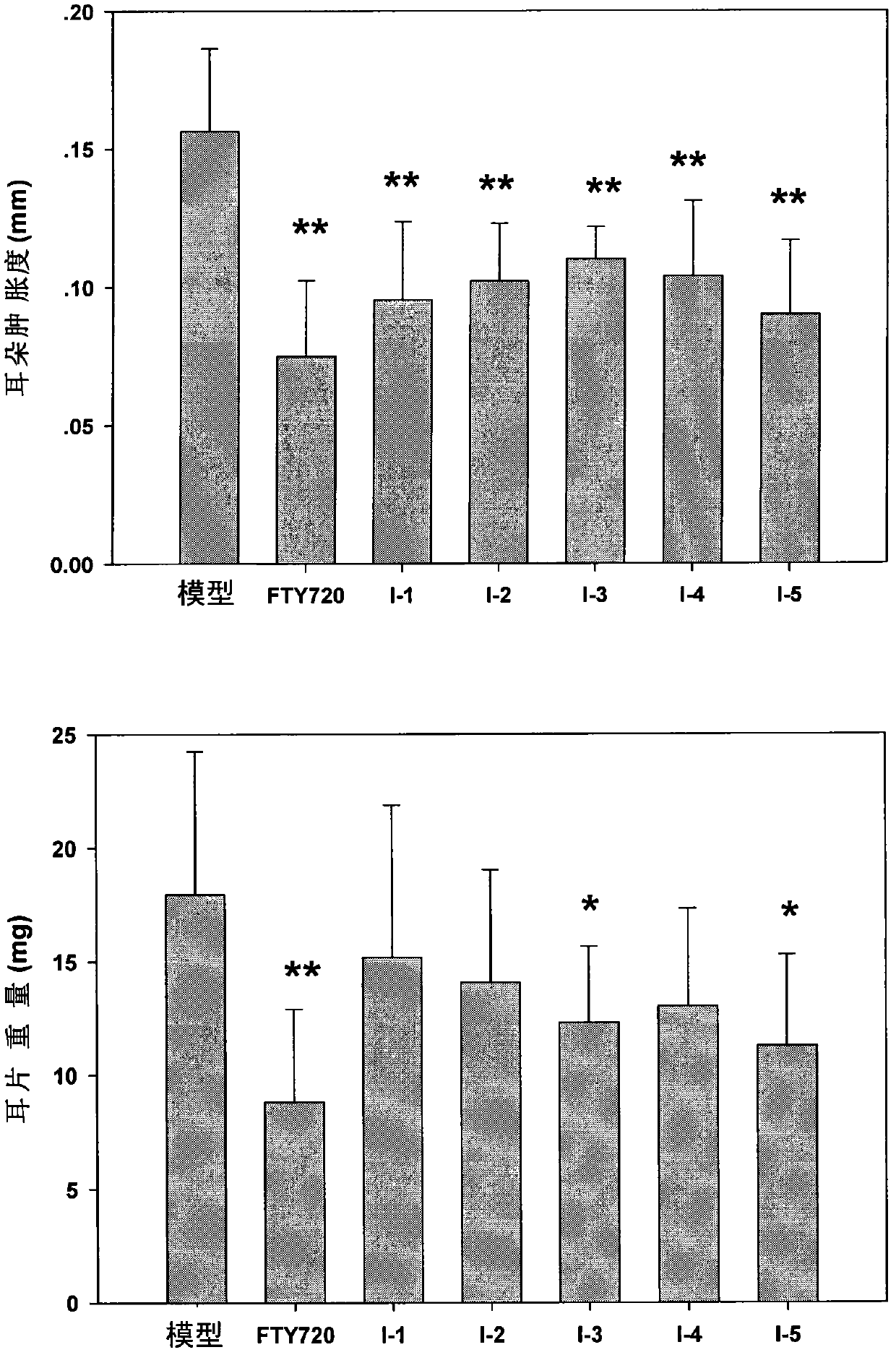
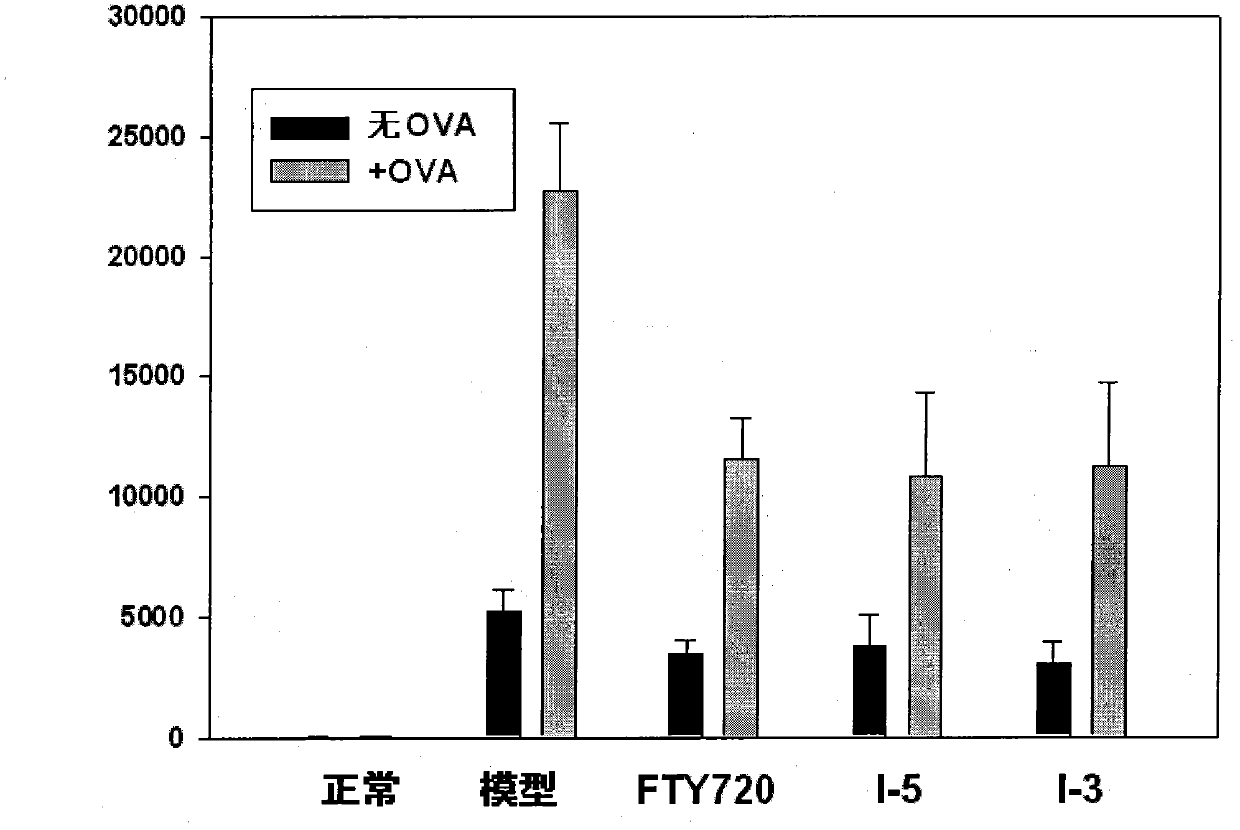
Immunomodulator and preparation method thereof as well as application thereof
A technology of immunomodulator and general formula, applied in the field of preparation of compounds, immunomodulators, new generation of immunomodulators or their pharmaceutically acceptable salts, can solve problems such as short-term bradycardia response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0059] 1) Methyl (E)-4-(4-n-butylcyclohexyl)benzoate (Ⅲ-4)
[0060] Dissolve (E)-4-(4-n-butylcyclohexyl)benzoic acid (II-4) (purchased from Shandong Yantai Fangchen Chemical Material Technology Co., Ltd.) (26.0g, 0.1mol) in 200mL methanol, dropwise 98% sulfuric acid (0.9 mL, 0.014 mol). After the dropwise addition, the mixture was heated under reflux for 8 hours, and the reaction was complete. After cooling, a white solid precipitated out, filtered with suction, washed with water, washed with a small amount of methanol, and dried at low temperature to obtain 24.2 g of a white solid, with a yield of 88%.
[0061] mp 39-40℃. 1 H NMR (500MHz, CDCl 3 ): δ0.90(t, 3H), 1.04-1.08(m, 2H), 1.23-1.25(m, 2H), 1.30-1.31(m, 5H), 1.44-1.47(m, 2H), 1.87-1.90 (m, 4H), 2.50-2.53(m, 1H), 3.90(s, 3H), 7.27(d, J=8Hz, 2H), 7.95(d, J=8Hz, 2H).
[0062] 2) Methyl 4-cyclohexylbenzoate (Ⅲ-1)
[0063] The method was the same as above, and 20.0 g of compound II-1 (purchased from Shandong Yantai Fan...
preparation Embodiment 2
[0075] 1) (E)-4-(4-n-butylcyclohexyl)benzyl alcohol (IV-4)
[0076] (E)-4-(4-n-butylcyclohexyl) methyl benzoate (Ⅲ-4) (27.4g, 0.1mol), potassium borohydride (6.5g, 0.12mol) and lithium chloride (5g, 0.12mol ) was suspended and dissolved in 100mL of anhydrous tetrahydrofuran, refluxed for 8 hours, cooled to room temperature, slowly added dropwise with 200mL of saturated ammonium chloride solution, continued to stir for 2 hours, concentrated under reduced pressure to remove tetrahydrofuran, extracted with ethyl acetate, and the organic phase was washed with water respectively, Wash with saturated sodium chloride solution, dry over anhydrous magnesium sulfate, and concentrate to obtain 22.2 g of white solid, yield: 90%.
[0077] mp 47-48°C. 1 H NMR (500MHz, CDCl 3 ): δ0.92(t, 3H), 1.07-1.08(m, 2H), 1.24-1.32(m, 7H), 1.46-1.48(m, 2H), 1.86-1.90(m, 4H), 2.48-2.50 (m, 1H), 4.66(s, 2H), 7.21(d, J=9Hz, 2H), 7.29(d, J=9Hz, 2H). 13 C NMR (CDCl 3 , 100MHz): δ23.0, 29.2, 33.6, 34.4, ...
preparation Embodiment 3
[0091] 1) (E)-4-(4-n-butylcyclohexyl)benzyl bromide (Ⅴ-4)
[0092] Dissolve (E)-4-(4-n-butylcyclohexyl)benzyl alcohol (IV-4) (34.5g, 0.14mol) in 200mL of dichloromethane, cool to -10°C in an ice bath, and drop 5.3 mL of phosphorus tribromide, reacted at room temperature for 6 hours, after the reaction was completed, crushed ice was added to destroy excess phosphorus tribromide, the organic phase was washed with water and saturated sodium chloride solution, dried over anhydrous sodium sulfate, and rotary evaporated to obtain a light yellow solid Product 41.5g, yield: 96%.
[0093] mp 33-35°C. 1 H NMR (500MHz, CDCl 3 ): δ0.92(t, 3H), 1.05-1.08(m, 2H), 1.25-1.34(m, 7H), 1.44-1.46(m, 2H), 1.88-1.90(m, 4H), 2.48-2.49 (m, 1H), 4.51(s, 2H), 7.20(d, J=9Hz, 2H), 7.33(d, J=9Hz, 2H). 13 C NMR (CDCl 3 , 100MHz): δ23.0, 29.2, 33.6, 33.7, 34.2, 37.1, 37.3, 44.4, 127.3, 129.0, 135.1, 148.3. MS (EI): m / z 308 (M + ,2).
[0094] 2) 4-cyclohexylbenzyl bromide (Ⅴ-1)
[0095] The method wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com