Quinoline and spirooxazine photochromic compound and preparation method thereof
A technology of photochromism and spirooxazine, applied in chemical instruments and methods, color-changing fluorescent materials, organic chemistry, etc., can solve the problems of fatigue resistance, color and low application range, achieve bright color, low preparation cost, The effect of fast fading speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
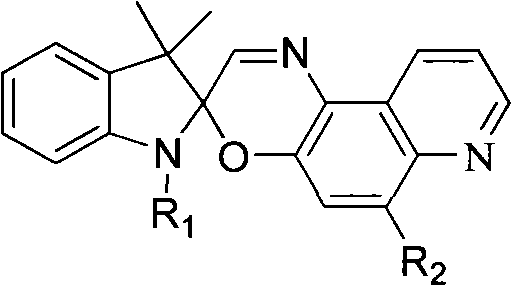
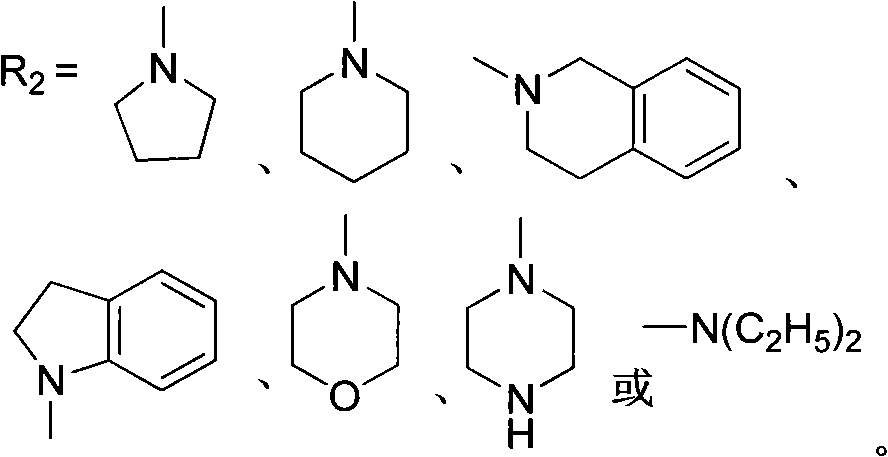
[0031] Preparation of 1,3,3-trimethyl-6'-morpholine-spiroindoline-2,3'-[3H]quinolino[2,1-b][1,4]oxazine photochromic compound.
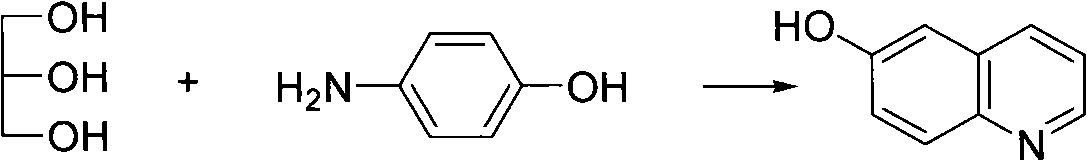
[0032] The first step, the synthesis of 6-hydroxyquinoline
[0033] In a 100mL four-necked bottle, add 3.39g of ferric sulfate heptahydrate, 10.9g of p-aminophenol and 8.35g of p-nitrophenol, and then add 6.2g of boric acid dissolved in 29mL of glycerin to the four-necked bottle, stir and heat up To 70°C, stop heating, dropwise add 13mL of sulfuric acid with a mass percentage concentration of 98%, continue heating to a boiling state for 8 hours, and adjust the pH value of the system to 5 with a 30% NaOH aqueous solution with a mass percentage concentration after the reaction. , the product 6-hydroxyquinoline was obtained by suction filtration, and the productive rate was 61%;
[0034] The second step, the synthesis of 5-nitroso-6-hydroxyquinoline
[0035] Dissolve 2 g of 6-hydroxyquinoline prepared in the first step in 30 mL of NaOH aqueous soluti...
Embodiment 2
[0042] Preparation of 1,3,3-trimethyl-6'-piperidine-spiroindoline-2,3'-[3H]quinolino[2,1-b][1,4]oxazine photochromic compound.
[0043] The first step, with embodiment 1;
[0044] Second step, with embodiment 1;
[0045] The third step, the synthesis of 5-nitroso-6-hydroxyquinoline Cu complex
[0046] Dissolve 2mmol of CuCl with stirring 2 10ml of the aqueous solution was added dropwise to 30mL of tetrahydrofuran and water mixed solution having 5mmol of 5-nitroso-6-hydroxyquinoline prepared in the second step, the volume ratio of tetrahydrofuran to water was 1:1, and continued After stirring for 10 minutes, the product 5-nitroso-6-hydroxyquinoline Cu complex was obtained by suction filtration with a yield of 98%.
[0047] The fourth step, 1,3,3-trimethyl-6'-piperidine-spiroindoline-2,3'-[3H]quinolino[2,1-b][1,4]oxazine Synthesis of Photochromic Compounds
[0048]
[0049] In a 50mL three-necked flask, add 1mmol of the 5-nitroso-6-hydroxyquinoline Cu complex prepared in ...
Embodiment 3
[0051] Preparation of 1,3,3-trimethyl-6'-piperazine-spiroindoline-2,3'-[3H]quinolino[2,1-b][1,4]oxazine photochromic compound.
[0052] The first step, with embodiment 1;
[0053] Second step, with embodiment 1;
[0054] The 3rd step, with embodiment 1;
[0055] The fourth step, 1,3,3-trimethyl-6'-piperazine-spiroindoline-2,3'-[3H]quinolino[2,1-b][1,4]oxazine Synthesis of Photochromic Compounds
[0056]
[0057] In a 50mL four-neck flask, add 1mmol of 5-nitroso-6-hydroxyquinoline Zn complex prepared in the third step, then add 15mL of ethanol solvent, then stir and add 3.5mmol of piperazine, stir and heat Refluxed for 2 hours, then added dropwise 1.5mmol 1,3,3-trimethyl-2-methylene indoline and 15mL ethanol solvent, refluxed for 8 hours, followed by TLC monitoring, and separated by recrystallization after the reaction was terminated to obtain a solid The product, namely 1,3,3-trimethyl-6'-piperazine-spiroindoline-2,3'-[3H]quinolino[2,1-b][1,4]oxazine Photochromic comp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com