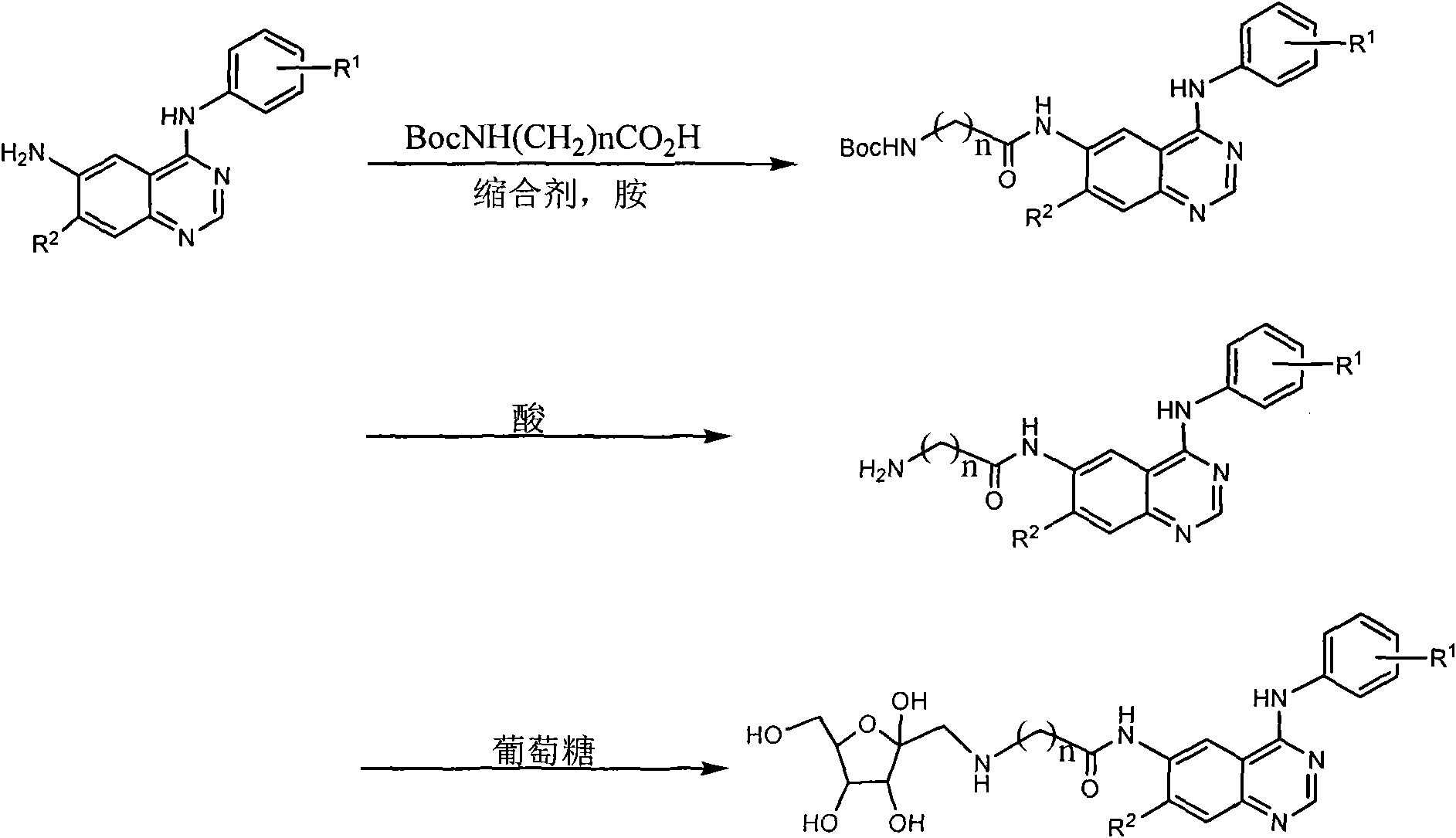
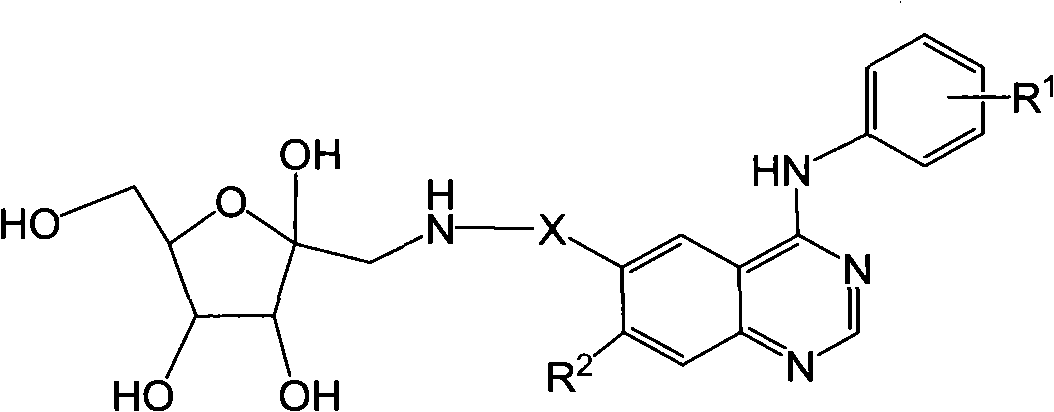
6-fructosamine-4-arylamidoquinazoline derivative and purpose thereof
A technology of arylaminoquinazoline and its derivatives, which is applied in the field of antineoplastic drugs and can solve the problems of low clinical efficacy and drug resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1: Preparation of 4-(3-chloro-4-fluoroanilino)-6-{2-[D-(1-deoxy-1-fructosylamino)]acetamido}quinazoline (A)
[0028] a) Preparation of intermediate 4-(3-chloro-4-fluoroanilino)-6-tert-butoxycarbonylaminoacetamidoquinazoline
[0029] Under nitrogen protection, 4-(3-chloro-4-fluoroanilino)-6-aminoquinazoline 0.98g, tert-butoxycarbonylaminoacetic acid 0.85g, diisopropylcarbodiimide 0.5mL, diiso A mixture of 0.8 mL of propylethylamine and 20 mL of tetrahydrofuran was reacted at 20° C. for 10 h, and the reaction mixture was separated by silica gel column chromatography (chloroform / methanol) to obtain 0.76 g of the product with a yield of 50.3%. MS: 446.2, 287.1.
[0030] b) Preparation of intermediate 4-(3-chloro-4-fluoroanilino)-6-aminoacetamidoquinazoline
[0031] A mixture of 4-(3-chloro-4-fluoroanilino)-6-tert-butoxycarbonylaminoacetamidoquinazoline 0.76g, dichloromethane 8mL and trifluoroacetic acid 2mL was stirred at room temperature for 6h, and saturated bic...
Embodiment 2
[0034] Example 2: 4-(3-chloro-4-fluoroanilino)-6-{2-[D-(1-deoxy-1-fructosylamino)]acetamido}quinazoline hydrochloride (B) preparation of
[0035] Add 40 mL of ethanol to 0.2 g of Compound A (the product obtained in step C in Example 1), shake it sufficiently to dissolve it, and add hydrogen chloride ethanol solution (about 2 mol L -1 ) 2mL, shake, stand still, filter with suction, and dry in the air to obtain 0.20g of a yellow solid. Yield 93.5%. MS: 508.1 (M+1), 317.1.
Embodiment 3
[0036] Example 3: 7-methoxy-4-[3-chloro-4-(3-fluorobenzyloxy)anilino]-6-{4-N-methyl-4-N-[D-(1 Preparation of -deoxy-1-fructose) amino] butyryl} amino quinazoline (C)
[0037] a) Intermediate 7-methoxy-4-[3-chloro-4-(3-fluorobenzyloxy)anilino]-6-(4-N-methyl-4-N-tert-butoxycarbonylamino The preparation of butyrylamino) quinazoline
[0038] Under nitrogen protection, 7-methoxy-4-[3-chloro-4-(3-fluorobenzyloxy)anilino]-6-aminoquinazoline 0.73g, 4-N-methyl-4- A mixture of 1.20 g of N-tert-butoxycarbonylaminobutyric acid, 1.1 g of dicyclohexylcarbodiimide, 0.5 mL of diisopropylethylamine and 20 mL of tetrahydrofuran was reacted at 20°C for 10 h, and the reaction mixture was separated by silica gel column chromatography (chloroform / methanol), to obtain product 0.84g, yield 79.3%. MS: 625.2 (M+1), 423.1.
[0039] b) Preparation of intermediate 7-methoxy-4-(3-chloro-4-fluoroanilino)-6-(4-N-methylaminobutanylamino)quinazoline
[0040] 7-methoxy-4-(3-chloro-4-fluoroanilino)-6-(4-N-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com