Preparation method of isopropyl 2-(3-nitrobenzylidene)acetoacetate
A technology of isopropyl acetate and nitrobenzylidene, applied in the field of medicine, can solve problems such as increasing the cost of industrialized production, unsatisfactory yield, etc., achieves improved yield and purity, shortened reaction time, and cheap and easy-to-obtain raw materials Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
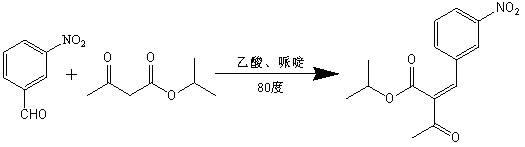
[0025] The preparation method of 2-(3-nitrobenzylidene) isopropyl acetoacetate of the present invention comprises the following steps:
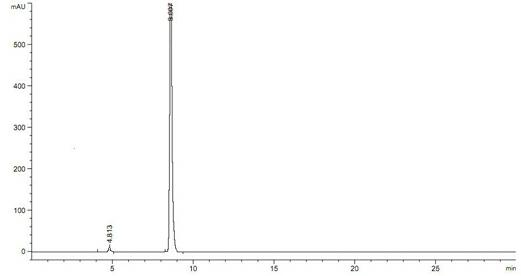
[0026] In the reaction bottle of 1000ml, add 151g (1mol) 3-nitrobenzaldehyde, 500ml isopropanol respectively, after stirring at room temperature, add 156g (1.1mol) isopropyl acetoacetate, then add 3g acetic acid, 6.6g piperidine, The reaction was stirred at 80°C and tracked with the liquid phase. After the reaction is completed, filter while it is hot (suction filtration), concentrate the filtrate in vacuum to 2 / 3-3 / 4 volume, then cool to below 0°C under stirring conditions to crystallize overnight. Filtrate with suction and wash the resulting solid with a small amount of isopropanol. Dry at 40-50°C (2 hours). After recrystallization with isopropanol, 267.9 g of the product was obtained, the yield was 91.4%, and mp: 92.5-94.8°C. Test results such as figure 1 Shown: After 4 hours, the reaction reaches the end point, and there is basically ...
Embodiment 2
[0030] According to the feeding amount and operating method of "Example 1", the experiments with reaction times of 2h, 4h, 5h, 6h, and 8h were done respectively, and all the other operations were the same. The results are as follows: at 2 hours, the yield was 65% (mp: 92.0~94.0℃); at 4 hours, the yield was 91.4% (mp: 93.0~94.8℃); at 5 hours, the yield was 91.0% (mp: 92.7~ 94.6℃); at 6 hours, the yield was 90.8% (mp: 92.5~94.9℃); at 8 hours, the yield was 90.5% (mp: 92.8~94.9℃). The results showed that: in the range of 2-4 h, the yield increased with time; at 4 h, the yield reached the highest; after 4 h, the yield basically remained unchanged.
Embodiment 3
[0032] With the charging amount and operation method of "Example 1" respectively with the molar ratio of acetic acid and piperidine mixture catalyst being 1:2, 1:1, 2:3, 2:1, experiments were done, and the yields of the reaction products were respectively as follows : 80.42% (mp: 92.7-94.9°C), 90.39% (mp: 92.9-94.8°C), 85.89% (mp: 93.1-94.8°C), 76.87% (mp: 92.8-95.1°C). The results show that: when using acetic acid and piperidine with a molar ratio of 1:1 as a catalyst, the reactant reacts most completely and the yield is also the highest; when using acetic acid and piperidine with a molar ratio higher or lower than 1:1 as a catalyst, The reactant reacts incompletely, so the yield is also low, and the fact also proves that there are many reactants in the reaction solution.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com