Application of hydrogenation catalyst to preparation of 1,4-cyclohexanediamine
A hydrogenation catalyst, cyclohexanediamine technology, applied in the preparation of amino compounds, metal/metal oxide/metal hydroxide catalysts, preparation of organic compounds, etc., can solve the problems of high catalyst consumption and high catalyst cost, Achieve the effect of reducing internal diffusion resistance, reducing catalyst cost and improving yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
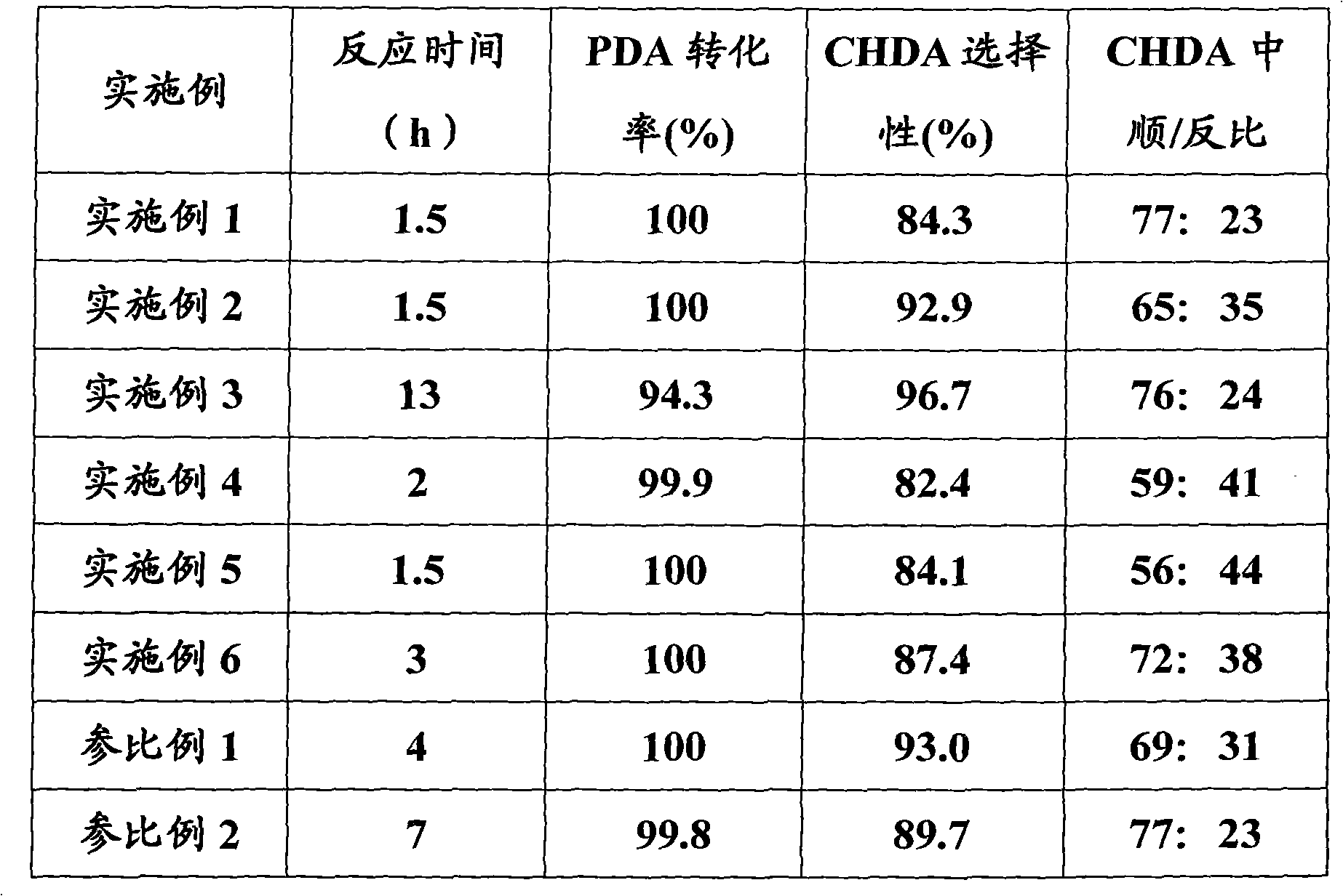
Examples
Embodiment 1
[0016] In a stirred autoclave, add 0.1 g of 10% Ru / mesoporous carbon catalyst (supported mesoporous carbon treated with 20% H 2 O 2 Pretreatment), p-phenylenediamine 5g, isopropanol 25ml, LiOH 0.05g, close the reactor, replace the air in the reactor with nitrogen three times, then replace the nitrogen in the reactor with hydrogen three times, then fill with hydrogen to make The reaction pressure of the reaction kettle reached 8.0 MPa, and the reaction kettle was heated to make the reaction temperature reach 120 ° C. The stirring was started, and the reaction was performed at a constant temperature until the pressure no longer decreased. The reaction product was taken out, the catalyst was removed by filtration, and analyzed by gas chromatography.
Embodiment 2
[0018] In an autoclave with stirring, add 10% Ru / mesoporous carbon catalyst 0.1g (support mesoporous carbon is pretreated with 6M hydrochloric acid), p-phenylenediamine 5g, isopropanol 25ml, LiOH 0.5g, close the reactor , replace the air in the reactor with nitrogen three times, and then replace the nitrogen in the reactor with hydrogen three times, and then fill with hydrogen to make the reaction pressure of the reactor reach 8.0MPa, heat the reactor to make the reaction temperature reach 120 ℃, start stirring, The reaction was carried out at a constant temperature until the pressure no longer decreased, the reaction product was taken out, the catalyst was removed by filtration, and analyzed by gas chromatography.
Embodiment 3
[0020] In a stirred autoclave, add 0.1 g of 10% Ru / mesoporous carbon catalyst (supported mesoporous carbon treated with 20% H 2 O 2 Pretreatment), p-phenylenediamine 5g, isopropanol 25ml, LiOH 0.5g, close the reactor, replace the air in the reactor with nitrogen three times, then replace the nitrogen in the reactor with hydrogen three times, then fill with hydrogen to make The reaction pressure of the reaction kettle reached 8.0MPa, the reaction kettle was heated to make the reaction temperature reach 80°C, stirring was started, and the reaction was performed at a constant temperature until the pressure no longer decreased, the reaction product was taken out, the catalyst was removed by filtration, and analyzed by gas chromatography.
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com