Rotigotine hydrochloride or free alkali film-forming gel preparation and preparation method thereof
A technology of gel preparation and hydrochloride, which is applied to medical preparations with non-active ingredients, medical preparations containing active ingredients, and pharmaceutical formulas, etc. It can solve the problem of large adverse reactions, instability, and low bioavailability of patches and other problems, to achieve the effect of good transdermal absorption, smooth gel, and good air permeability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
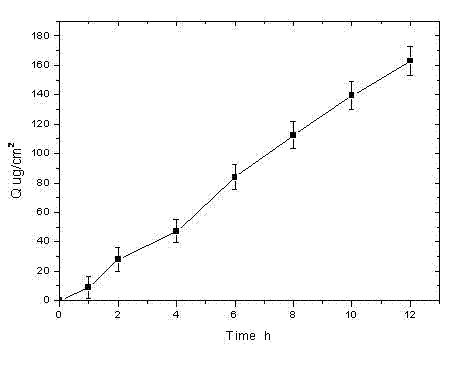
[0029] Rotigotine film-forming gel formulation
[0030] Prepare the gel (according to 100g) according to the following prescription:
[0031] Rotigotine free base 0.5g, hydroxypropyl cellulose 2.5g, carbomer 934 0.5g, silicone elastomer SF600A (Guangzhou Standard Beauty Company) 5.0g, glycerin 20.0g, triethanolamine 0.5g, ethanol 10ml, The balance is distilled water.
[0032] Weigh 0.5g of Carbomer 934 and 2.5g of hydroxypropyl cellulose, mix them evenly and sprinkle them into 60ml of deionized water for natural swelling. The next day, add 0.5g of triethanolamine to adjust the pH to neutral, weigh 0.5g of rotigotine free base and dissolve it in 10ml of absolute ethanol, stir to dissolve and slowly add it to the above gel dropwise, weigh 5.0g of silicone elastic Body SF600A (Guangzhou Biaomei Company), 20.0g glycerin, add water to make up to 100g, add it to the gel, stir and mix well, and get ready.
Embodiment 2
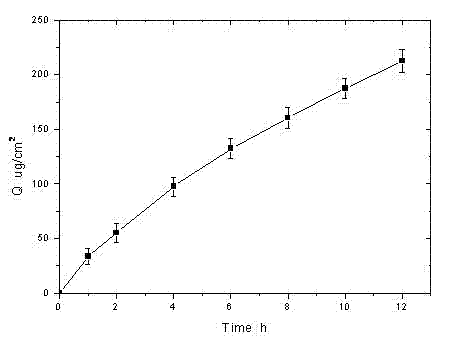
[0034] Rotigotine film-forming gel formulation
[0035] Prepare the gel (according to 100g) according to the following prescription:
[0036] Rotigotine free base 1.0g, hydroxyethylcellulose 0.5g, carbomer 934 10.0g, silicone elastomer ST-Elastomer 10 (Dow Corning Company) 3.0g, glycerin 15.0g, triethanolamine 5.0g, ethanol 15ml , and the balance is distilled water.
[0037] Weigh 10.0g of Carbomer 934 and 0.5g of hydroxyethyl cellulose, mix them evenly and sprinkle them into 60ml of deionized water for natural swelling. The next day, add an appropriate amount of triethanolamine (about 5.0g) to adjust the pH to neutral, weigh 1.0g of rotigotine free base and dissolve it in 15ml of absolute ethanol, stir and dissolve, slowly add it to the above gel, and weigh 3.0 g silicone elastomer ST-Elastomer 10 (Dow Corning Company), glycerin 15.0g, add water to make up to 100g, add to the gel, stir and mix well to obtain.
Embodiment 3
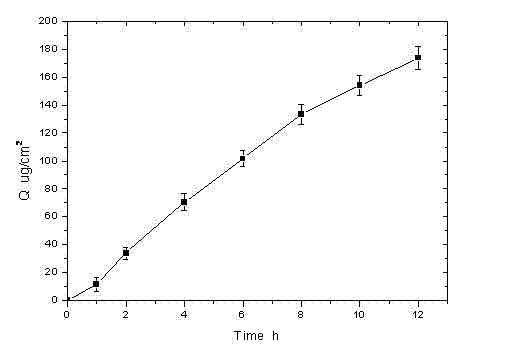
[0039] Rotigotine film-forming gel formulation
[0040]Prepare the gel (according to 100g) according to the following prescription:
[0041] Rotigotine Free Base 2.0g, Hypromellose 1.0g, Carbomer 940 9.0g, Silicone Elastomer ST-Elastomer 10 (Dow Corning Company) 2.0g, Glycerin 5.0g, Triethanolamine 4.0g, Ethanol 18ml, the balance is distilled water.
[0042] Weigh 9.0g of Carbomer 934 and 1.0g of hydroxypropyl methylcellulose, mix them evenly and sprinkle them into 60ml of deionized water for natural swelling. The next day, add an appropriate amount of triethanolamine (about 4.0g) to adjust the pH to neutral, weigh 2.0g of rotigotine free base and dissolve it in 18ml of absolute ethanol, stir to dissolve and slowly add it to the above gel, weigh 2.0 g silicone elastomer ST-Elastomer 10 (Dow Corning Company), add water to 5.0 g of glycerin to make up to 100 g, add it to the gel, stir and mix well, and obtain.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com