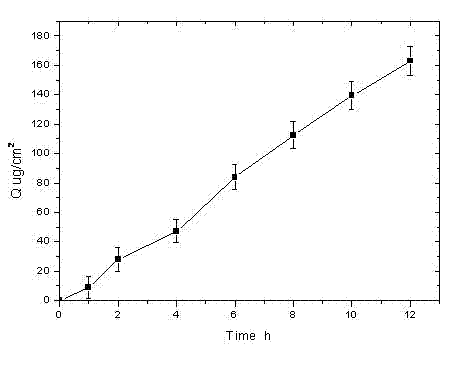
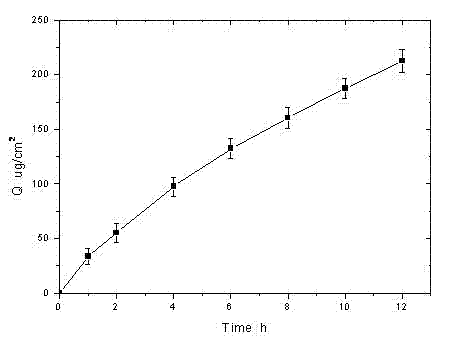
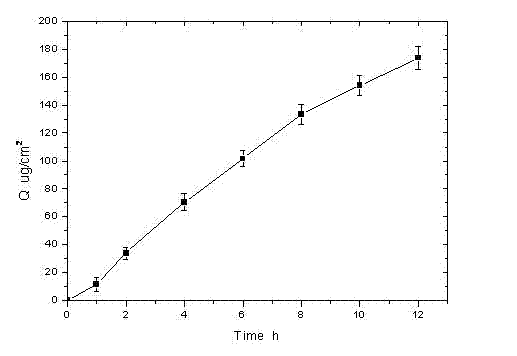
Rotigotine hydrochloride or free alkali film-forming gel preparation and preparation method thereof
A technology of gel preparation and hydrochloride, which is applied to medical preparations with non-active ingredients, medical preparations containing active ingredients, and pharmaceutical formulas, etc., which can solve the problem of large adverse reactions, instability, and low bioavailability of patches and other problems, to achieve the effect of good transdermal absorption, smooth gel, and good air permeability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Rotigotine film-forming gel formulation
[0030] Prepare the gel (by 100g) according to the following recipe:
[0031] Rotigotine Free Base 0.5g, Hydroxypropyl Cellulose 2.5g, Carbomer 934 0.5g, Silicone Elastomer SF600A (Guangzhou Biaomei Company) 5.0g, Glycerin 20.0g, Triethanolamine 0.5g, Ethanol 10ml, The remainder is distilled water.
[0032] Weigh 0.5g of carbomer 934 and 2.5g of hydroxypropyl cellulose, mix and sprinkle into 60ml of deionized water to swell naturally. The next day, add 0.5g triethanolamine to adjust the pH to neutrality, weigh 0.5g rotigotine free base and dissolve it in 10ml absolute ethanol, stir and dissolve, slowly add it dropwise to the above gel, weigh 5.0g silicone elastic Body SF600A (Guangzhou Biaomei Co., Ltd.), 20.0g glycerin, add water to make up to 100g, add it to the gel, stir and mix well, that's it.
Embodiment 2
[0034] Rotigotine film-forming gel formulation
[0035] Prepare the gel (by 100g) according to the following recipe:
[0036] Rotigotine free base 1.0g, hydroxyethyl cellulose 0.5g, carbomer 934 10.0g, silicone elastomer ST-Elastomer 10 (Dow Corning) 3.0g, glycerin 15.0g, triethanolamine 5.0g, ethanol 15ml , the remainder is distilled water.
[0037] Weigh 10.0g of carbomer 934 and 0.5g of hydroxyethyl cellulose, mix and evenly sprinkle it into 60ml of deionized water to swell naturally. The next day, an appropriate amount of triethanolamine (about 5.0 g) was added to adjust the pH to neutrality. 1.0 g of rotigotine free base was weighed and dissolved in 15 ml of absolute ethanol. After stirring and dissolving, it was slowly added dropwise to the above gel. g silicone elastomer ST-Elastomer 10 (Dow Corning Corporation), 15.0 g of glycerin, add water to make up to 100 g, add it to the gel, stir and mix, and that’s it.
Embodiment 3
[0039] Rotigotine film-forming gel formulation
[0040]Prepare the gel (by 100g) according to the following recipe:
[0041] Rotigotine Free Base 2.0g, Hypromellose 1.0g, Carbomer 940 9.0g, Silicone Elastomer ST-Elastomer 10 (Dow Corning) 2.0g, Glycerin 5.0g, Triethanolamine 4.0g, Ethanol 18ml, the balance is distilled water.
[0042] Weigh 9.0g of carbomer 934 and 1.0g of hydroxypropyl methylcellulose, mix and sprinkle into 60ml of deionized water to swell naturally. The next day, an appropriate amount of triethanolamine (about 4.0 g) was added to adjust the pH to neutrality, and 2.0 g of rotigotine free base was weighed and dissolved in 18 ml of absolute ethanol. g silicone elastomer ST-Elastomer 10 (Dow Corning), add 5.0 g of glycerol, add water to make up to 100 g, add it to the gel, stir and mix well, and that’s it.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com