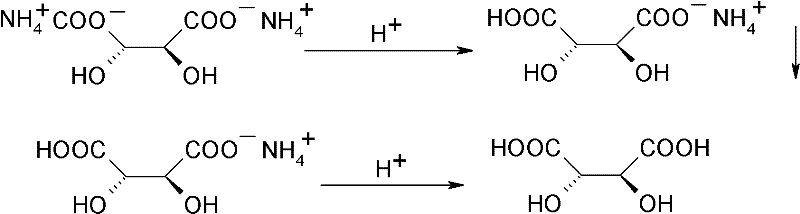
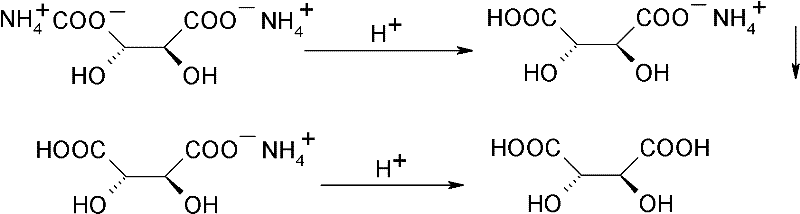
Method for recovering and recycling L-tartaric acid
A technology of tartaric acid and ammonium hydrogen tartrate, applied in chemical instruments and methods, preparation of carboxylates, preparation of organic compounds, etc., can solve the problems of high cost and large energy consumption, and achieves reduced energy consumption, simple process operation, and reaction mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] (1) acidification precipitation: get the waste water 1000g (7wt% L-tartaric acid) containing diammonium tartrate that the p-thymphenyl phenylserine ethyl ester resolution process of racemization produces, be 45-50 ℃ at reaction temperature, with concentrated Hydrochloric acid was acidified to pH 3, and the reaction time was 0.5h. The reaction solution formed a precipitate of ammonium hydrogen tartrate and was filtered. The obtained precipitate was dried and dehydrated to obtain 66.5 g of ammonium hydrogen tartrate (85.3% recovery rate). L-ammonium hydrogen tartrate is an off-white solid with a purity greater than 99% and an optical purity of 100% as determined by HPLC.
[0024] (2) Acidification desalination: get the product ammonium hydrogen tartrate 33.4g obtained in the step (1), add 135g methyl alcohol, add the concentrated sulfuric acid of 10g, carry out acidification reaction. The reaction temperature is 42-45°C, and the reaction time is 2h. After the reaction,...
Embodiment 2
[0026] (1) Acidification precipitation: get the waste water 1000g (8wt% L-tartaric acid) containing the diammonium tartrate that the racemized p-thymphenylphenylserine ethyl ester resolution process produces, be 0 ℃ at reaction temperature, acidify with concentrated sulfuric acid To pH 2.5, the reaction time is 2h. The reaction solution formed a precipitate of ammonium hydrogen tartrate and was filtered. The obtained precipitate was dried and dehydrated to obtain 81.9 g of ammonium hydrogen tartrate (92.0% recovery). L-ammonium hydrogen tartrate is an off-white solid with a purity greater than 99% and an optical purity of 100% as determined by HPLC.
[0027] (2) acidification desalination: get the product ammonium hydrogen tartrate 33.4g of gained in the step (1), add 135g methyl alcohol, add 23.1g hydrochloric acid methanol solution (30%), carry out acidification reaction. The reaction temperature is 20-25°C, and the reaction time is 6h. After the reaction, the inorganic s...
Embodiment 3
[0029] (1) Acidification precipitation: get the waste water 1000g (10wt% L-tartaric acid) containing the diammonium tartrate that the racemized p-thymphenylphenylserine ethyl ester resolution process produces, be 25-30 ℃ at reaction temperature, with phosphoric acid Acidification to pH 4.0-4.5, the reaction time is 4h. The reaction solution formed a precipitate of ammonium hydrogen tartrate and was filtered. The obtained precipitate was dried and dehydrated to obtain 92.4 g of ammonium hydrogen tartrate (recovery rate: 83.0%). L-ammonium hydrogen tartrate is an off-white solid with a purity greater than 99% and an optical purity of 100% as determined by HPLC.
[0030] (2) acidification desalination: get the product ammonium hydrogen tartrate 33.4g obtained in the step (1), add 135g methyl alcohol, add 12g acetic acid, carry out acidification reaction. The reaction temperature is 0°C, and the reaction time is 10 h. After the reaction, the inorganic salts are removed by filtr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com