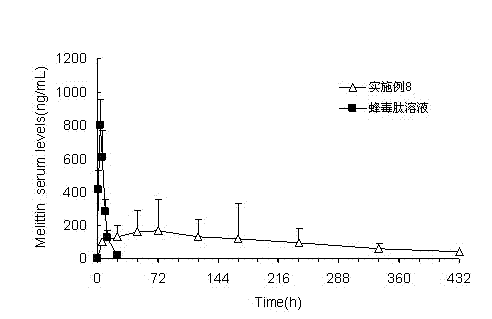
Melittin pamoate capable of being continuously released and preparation agent thereof
A technology of pamolate and pamoate sodium salt, applied in the field of medicine, can solve problems such as biological activity influence, and achieve the effect of short half-life and unstable physical and chemical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
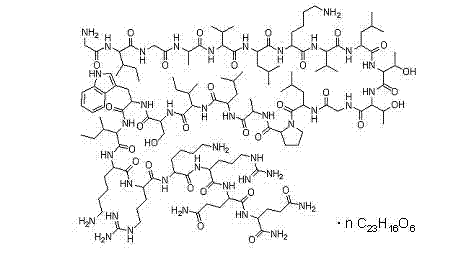
[0023] The separation and purification of embodiment 1 melittin
[0024] Weigh 10g of dried bee venom, put it in a centrifuge tube, add formic acid-ammonium formate (0.1M, pH4.5) buffer solution to fully dissolve it, centrifuge at 4000r / min for 5min, absorb the supernatant, pass through a 0.45um micropore Membrane filtration; wash the residue at the bottom of the centrifuge tube with buffer, centrifuge, take the supernatant, filter, combine the two filtrates, and set aside. Put the above-mentioned pretreated sample on a Sephadex G-50 column, and use formic acid-ammonium formate buffer as the eluent for elution, the flow rate is 10mL / h, and the detection wavelength is 280nm; The column was subjected to secondary chromatography under the same conditions, and the eluate from the main peak was collected and freeze-dried. The purity of melittin isolated by high performance gel permeation chromatography was 96.4%.
Embodiment 2
[0025] Example 2 Preparation of pamoic acid sodium salt solution (0.1M)
[0026] Suspend 3.88g of pamoic acid in 20ml of water for injection, add 20ml of 1.0M sodium hydroxide solution under stirring until the pamoic acid is completely dissolved, add water for injection to dilute to 100ml.
Embodiment 3
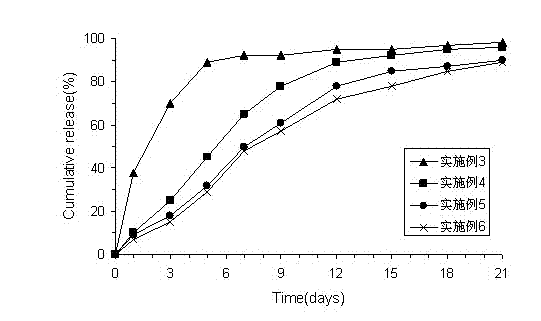
[0027] Example 3 Preparation of melittin pamolate (1:0.5 mol / mol)
[0028] Dissolve 1.42g of melittin in 20ml of water for injection, and add 10ml of pamoic acid disodium salt solution (0.1M) under stirring until crystallization occurs. The precipitate was filtered off, washed with water for injection and dried. The product contained 2:1 peptide pamolate (mol / mol).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com