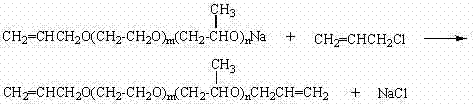
Method for synthetizing diallyl polyether
The technology of a bisallyl polyether and its synthesis method is applied in the field of synthesis of bisallyl polyether, which can solve the problems of low capping rate and poor reactivity, and achieve high capping rate, low cost and excellent production process. simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
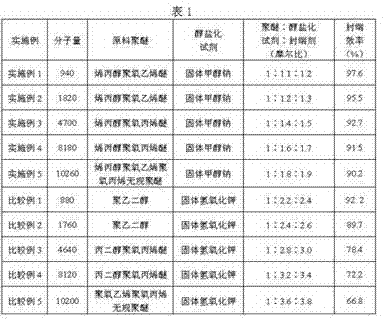
[0034] Add 1,000 grams of allyl alcohol polyoxyethylene ether (m=20, n=0, molecular weight about 940) and 63.2 grams of solid sodium methoxide into a 2L reactor, and stir vigorously. The temperature is 90~100℃, under vacuum condition (pressure -0.09~-0.1MPa) to remove methanol, and react for 3 hours. Cool down to 60-70°C, slowly introduce 97.7 g of allyl chloride, maintain the pressure at 0.3 MPa, and continue the reaction for 4 hours. Excess allyl chloride is recovered by distillation under reduced pressure, and the end-capped polyether product is obtained after filtering to remove solid impurities. This embodiment solves the problems of poor reactivity and low end capping rate of high molecular weight polyether. The selection of the alkoxide reagent not only solves the problem of the by-product of the highly toxic allyl alcohol in the conventional allylation process, but also makes the product have a higher end-capping rate.
Embodiment 2
[0036] Add 1,000 grams of allyl alcohol polyoxyethylene ether (m=40, n=0, molecular weight about 1820) and 35.6 grams of sodium methoxide into a 2L reactor, and stir vigorously. The temperature is 100~110℃, under vacuum conditions (pressure -0.09~-0.1MPa) to remove methanol, and react for 3 hours. Cool down to 60-70°C, slowly introduce 54.6 g of allyl chloride, maintain the pressure at 0.3 Mpa, and continue the reaction for 4 hours. Excess allyl chloride is recovered by distillation under reduced pressure, and the end-capped polyether product is obtained after filtering to remove solid impurities. This embodiment solves the problems of poor reactivity and low end capping rate of high molecular weight polyether. The selection of the alkoxide reagent not only solves the problem of the by-product of the highly toxic allyl alcohol in the conventional allylation process, but also makes the product have a higher end-capping rate.
Embodiment 3
[0038] Add 1,000 grams of allyl alcohol polyoxypropylene ether (m=0, n=80, molecular weight about 4700), 16.1 grams of solid sodium methoxide into a 2L reactor, and stir vigorously. The temperature is 110~120℃, under vacuum condition (pressure -0.09~-0.1MPa) to remove methanol, and react for 3 hours. Cool down to 80-90°C, slowly introduce 24.4 g of allyl chloride, maintain the pressure at 0.3 Mpa, and continue the reaction for 5 hours. Excess allyl chloride is recovered by distillation under reduced pressure, and the end-capped polyether product is obtained after filtering to remove solid impurities. This embodiment solves the problems of poor reactivity and low end capping rate of polyethers containing secondary hydroxyl groups, especially high molecular weight polyethers. The selection of the alkoxide reagent not only solves the problem of the by-product of the highly toxic allyl alcohol in the conventional allylation process, but also makes the product have a higher end-ca...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com