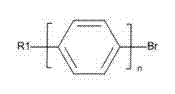
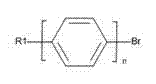
Synthetic method of cyano-biphenyl type liquid crystal material
A technology of liquid crystal materials and synthesis methods, which is applied in the direction of liquid crystal materials, chemical instruments and methods, and the preparation of organic compounds. It can solve the problems of low yield, many by-products, and expensive organic lithium reagents, and achieve simple post-reaction treatment. , simple reaction operation, reduced equipment investment and production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1: Synthesis of 4'-pentyloxy-4-cyanodiphenyl
[0027] Under nitrogen protection, 20 mmol of 4-bromobenzonitrile, 0.1 mmol of palladium acetate, 0.12 mmol of Ph 3 P and 2 mmol of zinc bromide were dissolved in 30 ml of THF, and then 50 ml of Grignard reagent prepared from 20 mmol of 4-pentyloxybromobenzene was added dropwise. After the addition was completed, the mixture was reacted at room temperature for 2 hours. Afterwards, the reaction solution was cooled in an ice-water bath, and then 50 ml of 1% by mass dilute saline solution was added dropwise, stirred for 30 minutes after the dropwise addition, and the organic phase was separated, and the aqueous phase was extracted twice with 50 ml of ethyl acetate, and the organic phases were combined , and washed twice with 50 ml of water, the solvent was evaporated in a rotary evaporator to obtain a crude product. The crude product was recrystallized with methanol and decolorized with activated carbon to obtain pure ...
Embodiment 2
[0029] Example 2: Synthesis of 4'-pentyloxy-4-cyanodiphenyl
[0030] Under nitrogen protection, 20 mmol of 4-bromobenzonitrile, 0.2 mmol of palladium acetate, 0.24 mmol of Ph 3 P and 3 mmol of zinc bromide were dissolved in 30 ml of THF, and then 50 ml of Grignard reagent prepared from 20 mmol of 4-pentyloxybromobenzene was added dropwise. After the addition was completed, the reaction was carried out at room temperature for 2 hours. Afterwards, the reaction solution was cooled in an ice-water bath, and then 50 ml of 1% by mass dilute saline solution was added dropwise, stirred for 30 minutes after the dropwise addition, and the organic phase was separated, and the aqueous phase was extracted twice with 50 ml of ethyl acetate, and the organic phases were combined , and washed twice with 50 ml of water, the solvent was evaporated in a rotary evaporator to obtain a crude product. The crude product was recrystallized with methanol and decolorized with activated carbon to obtain ...
Embodiment 3
[0031] Example 3: Synthesis of 4'-pentyloxy-4-cyanobiphenyl
[0032] Under nitrogen protection, 20 mmol of 4-bromobenzonitrile, 0.4 mmol of palladium acetate, 0.48 mmol of Ph 3P and 4 mmol of zinc bromide were dissolved in 30 ml of THF, and then 50 ml of Grignard reagent prepared from 20 mmol of 4-pentyloxybromobenzene was added dropwise. After the addition was completed, the mixture was reacted at room temperature for 2 hours. Afterwards, the reaction solution was cooled in an ice-water bath, and then 50 ml of 1% by mass dilute saline solution was added dropwise, stirred for 30 minutes after the dropwise addition, and the organic phase was separated, and the aqueous phase was extracted twice with 50 ml of ethyl acetate, and the organic phases were combined , and washed twice with 50 ml of water, the solvent was evaporated in a rotary evaporator to obtain a crude product. The crude product was recrystallized with methanol and decolorized with activated carbon to obtain pure 4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com