Product of Maillard reaction of tryptophan and glucose and its preparation method and application
A technology of Maillard reaction and glucose, applied in the field of medicine, can solve the problem of no tumor inhibitory effect and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
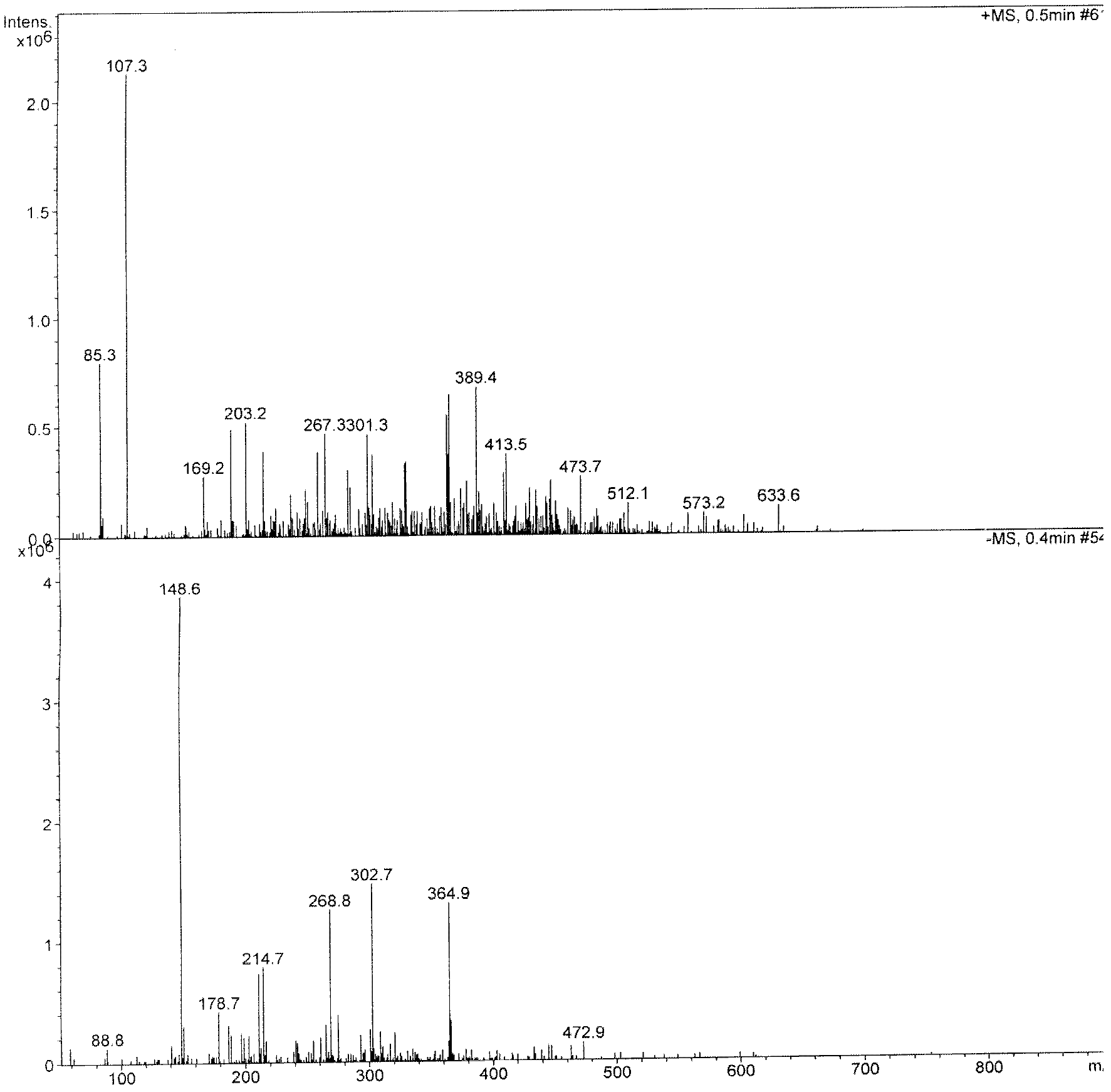
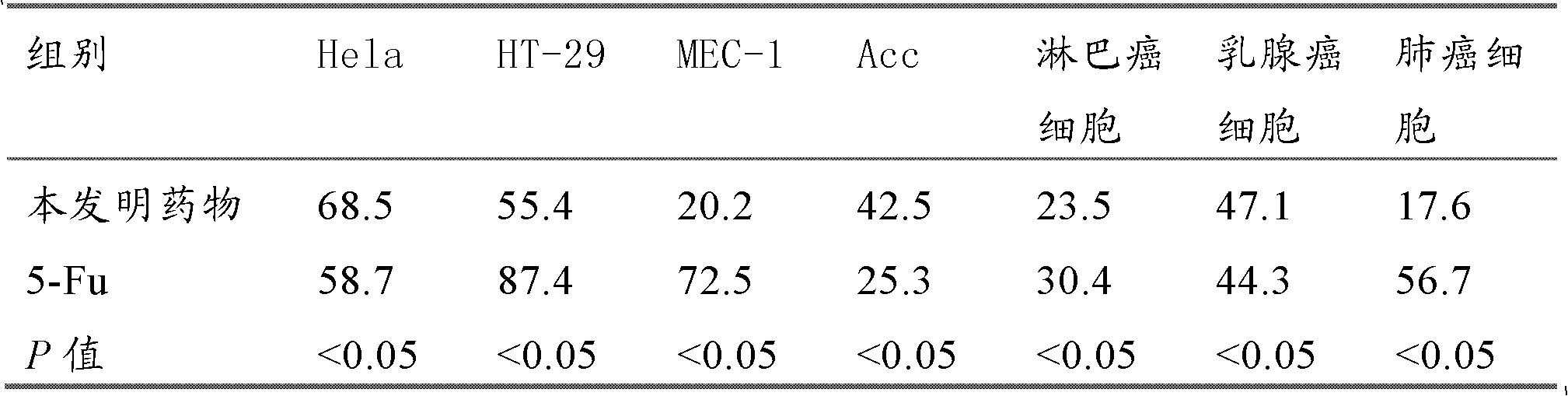
Image
Examples
Embodiment 1
[0020] Synthesis of the Maillard product of embodiment 1 tryptophan and glucose
[0021] Raw materials: L-tryptophan was purchased from SIGMA; D-glucose was purchased from Tianjin Fengchuan Chemical Reagent Technology Co., Ltd.
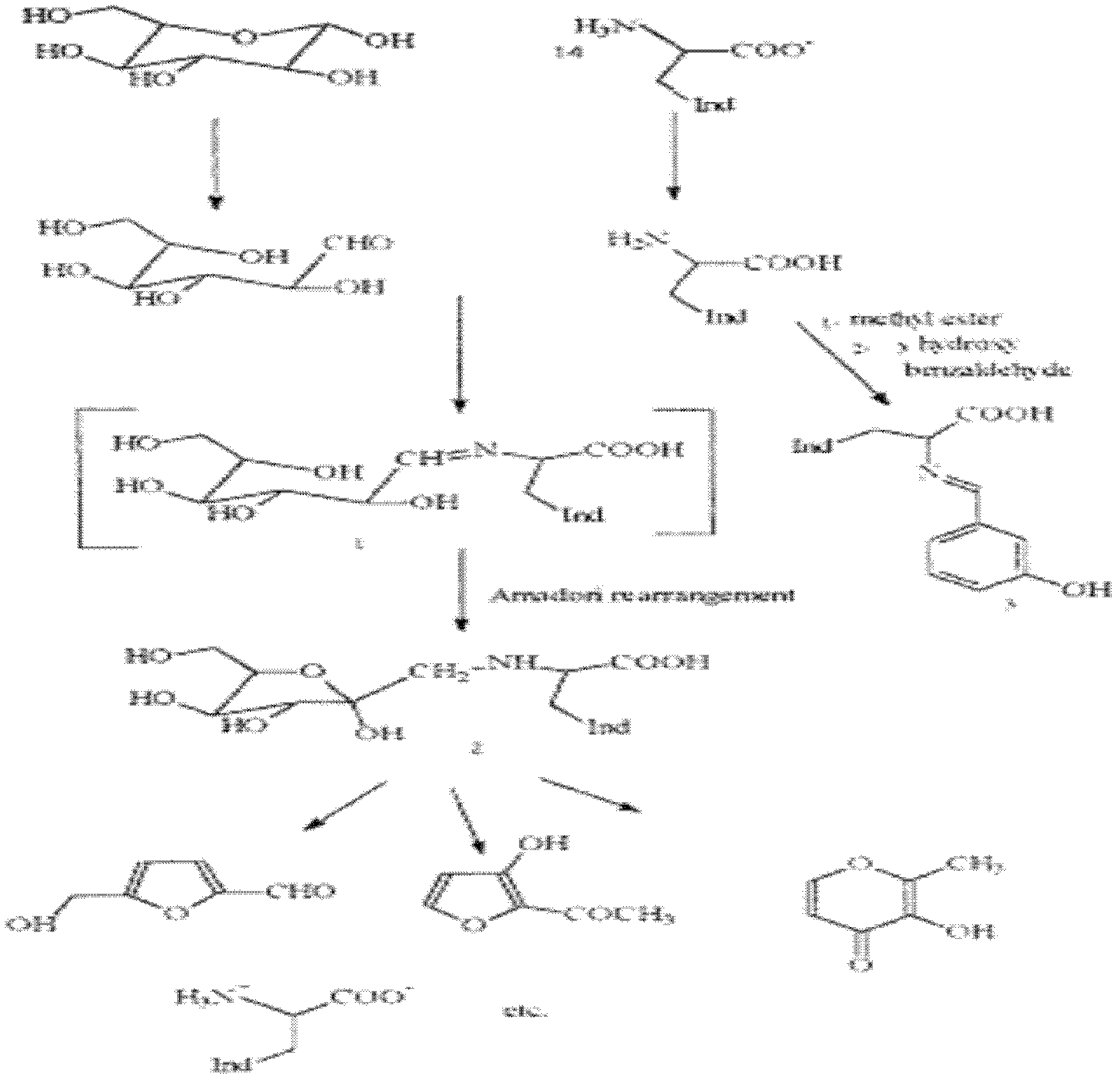
[0022] Synthesis of Maillard products of tryptophan and glucose: as figure 1 As shown, take 5 g of L-tryptophan and 40 g of D-glucose and add it to 600 ml of anhydrous methanol, heat and reflux for 5 hours, and evaporate to dryness under reduced pressure at 40 degrees Celsius to 50 ml, add 8 ml of water, and add the resulting solution to the cellulose ( Whatman company, in the 44 * 550mm chromatographic column that CF-11 cellulose) fills, use the n-butanol solution saturated with water to carry out elution, obtain 20ml eluate, vacuum freeze-dry, obtain target product-brown yellow solid 2- 5g.
Embodiment 2
[0023] Synthesis of the Maillard product of embodiment 2 tryptophan and glucose
[0024] Raw materials: L-tryptophan was purchased from SIGMA; D-glucose was purchased from Tianjin Fengchuan Chemical Reagent Technology Co., Ltd.
[0025] Dissolve 0.58g of potassium hydroxide in 12ml of methanol, then add 3g of L-tryptophan, stir to completely dissolve the tryptophan, then add 15ml of methanol solution containing 1.5g of D-glucose. The above solution was stirred and heated to reflux at 70° C. for 2 h. After the reaction, the solution was cooled to room temperature, and the solution was concentrated to 10 ml using a vacuum rotary evaporator, filtered with suction to remove excess amino acids, and the solution was cooled to 0°C. Add acetone dropwise to the solution until no precipitation occurs, and filter with suction to obtain an amorphous yellow powder. The yellow powder was redissolved in methanol, acetone was added dropwise to make it precipitate, and it was suction filtere...
Embodiment 3
[0026] Synthesis of the Maillard product of embodiment 3 tryptophan and glucose
[0027] Raw materials: L-tryptophan was purchased from SIGMA; D-glucose was purchased from Tianjin Fengchuan Chemical Reagent Technology Co., Ltd.
[0028] Materials: Dowex 50W-X8H+ column (10*2cm) was purchased from Dow Company, USA.
[0029] Add 1g of L-tryptophan and 9g of D-glucose into 150ml of chromatographically pure methanol and heat to reflux for 5h. The mixture is concentrated under reduced pressure, dissolved in 50ml of double distilled water, and the sample is added to a Dowex 50W-X8H+ column (10*2cm), Use 200ml of water as the mobile phase for elution (to elute excess glucose). The product was eluted with 150 ml of 1 mol / L ammonia water, and the eluent was rotary evaporated to obtain a crude product. RP-HPLC was used for separation, and the mobile phase was methanol:water=65:35, which contained 0.1% TFA. The product-containing eluate was dried under reduced pressure. The obtained ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com