Preparation method for high purity etofenamate
An etofenamate, high-purity technology, applied in the field of drug synthesis, can solve the problems of poor product appearance, difficult separation, low content, etc., and achieve the effect of good appearance, simple separation and purification, and high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
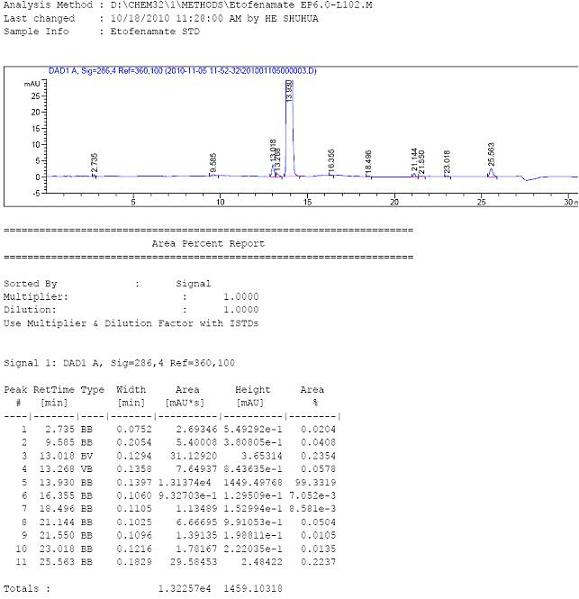
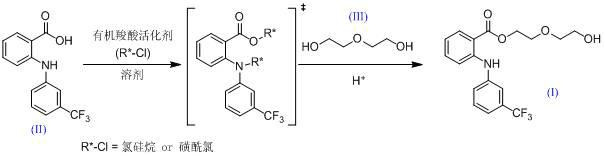
[0036] Embodiment 1: the preparation of etofenamate.
[0037] Add 281.5 g of flufenamic acid into 1000 g of toluene, stir and cool to 10°C, add 217.5 g of trimethylchlorosilane (R*=TMS), and stir at this temperature for 1 hour. Then 1062 grams of diethylene glycol was added, and the temperature was raised to 30° C. to continue the reaction for 5 hours. The reaction process was tracked and detected by TLC or HPLC until the raw materials and intermediates of flufenamic acid disappeared, then 800 g of 10% dilute hydrochloric acid was added and stirred and hydrolyzed at 30-40°C for 1 hour, and it was tracked and detected by HPLC until it was completely converted into etofenamate. The reaction liquid was cooled to about 10°C, and then the pH of the reaction system was adjusted to about 9 with sodium carbonate, and the layers were separated after standing. The organic layer was washed five times with 1000 g of pure water, then decolorized by adding an appropriate amount of activate...
Embodiment 2
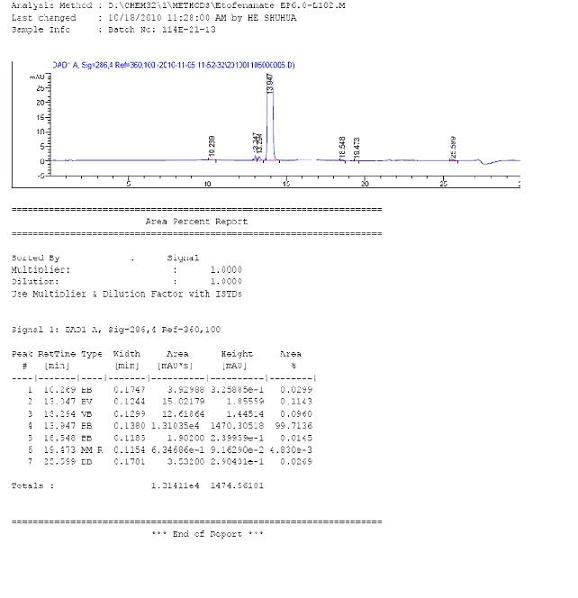
[0038] Embodiment 2: the preparation of etofenamate.
[0039] Add 281.5 g of flufenamic acid into 1000 g of toluene, stir and cool to 10°C, add 331.6 g of tert-butyldimethylsilyl chloride (R*=TBDMS), and keep stirring at 20°C for 1 hour. Then add 1592 grams of diethylene glycol and heat up to 40°C to continue the reaction for 5 hours. The reaction process was tracked and detected by TLC or HPLC until the raw materials and intermediates of flufenamic acid disappeared, then 900 g of 10% dilute hydrochloric acid was added and stirred and hydrolyzed at 30-40°C for 1 hour, and it was tracked and detected by HPLC until it was completely converted into etofenamate. The reaction solution was cooled to about 10°C, and then the pH of the reaction system was adjusted to about 9 with potassium carbonate, and the mixture was allowed to stand for stratification. The organic layer was washed six times with 1200 g of pure water, then added an appropriate amount of active carbon for decolori...
Embodiment 3
[0040] Embodiment 3: the preparation of etofenamate.
[0041] Add 281.5 g of flufenamic acid into 1200 g of DMF, stir and cool to 10°C, add 381.5 g of p-toluenesulfonyl chloride (R*=Ts), and keep stirring at 20°C for 1 hour. Then add 1062 grams of diethylene glycol, and heat up to 40°C to continue the reaction for 5 hours. Use TLC or HPLC to track and detect the disappearance of flufenamic acid raw materials and intermediates. Concentrate the reaction solution under reduced pressure at 50~60°C to about 1700ml, then add 800g of 10% dilute hydrochloric acid, stir and hydrolyze at 30~40°C for 2 hours , HPLC tracking detection to all converted to etofenamate. The reaction solution was cooled to about 10°C, and then the pH of the reaction system was adjusted to about 10 with sodium carbonate, and the reaction solution was extracted twice with 1000 grams of toluene. The organic layers were combined, washed five times with 1000 g of pure water, then decolorized by adding an approp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com