Method for refining crude lead
A technology of crude lead and electrolytic lead, applied in the fields of instruments, optics, photographic technology, etc., can solve the problems such as failure to develop electrolysis in alkaline system, limited solubility, easy volatility, etc. The effect of low power consumption and low electrolytic cell pressure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
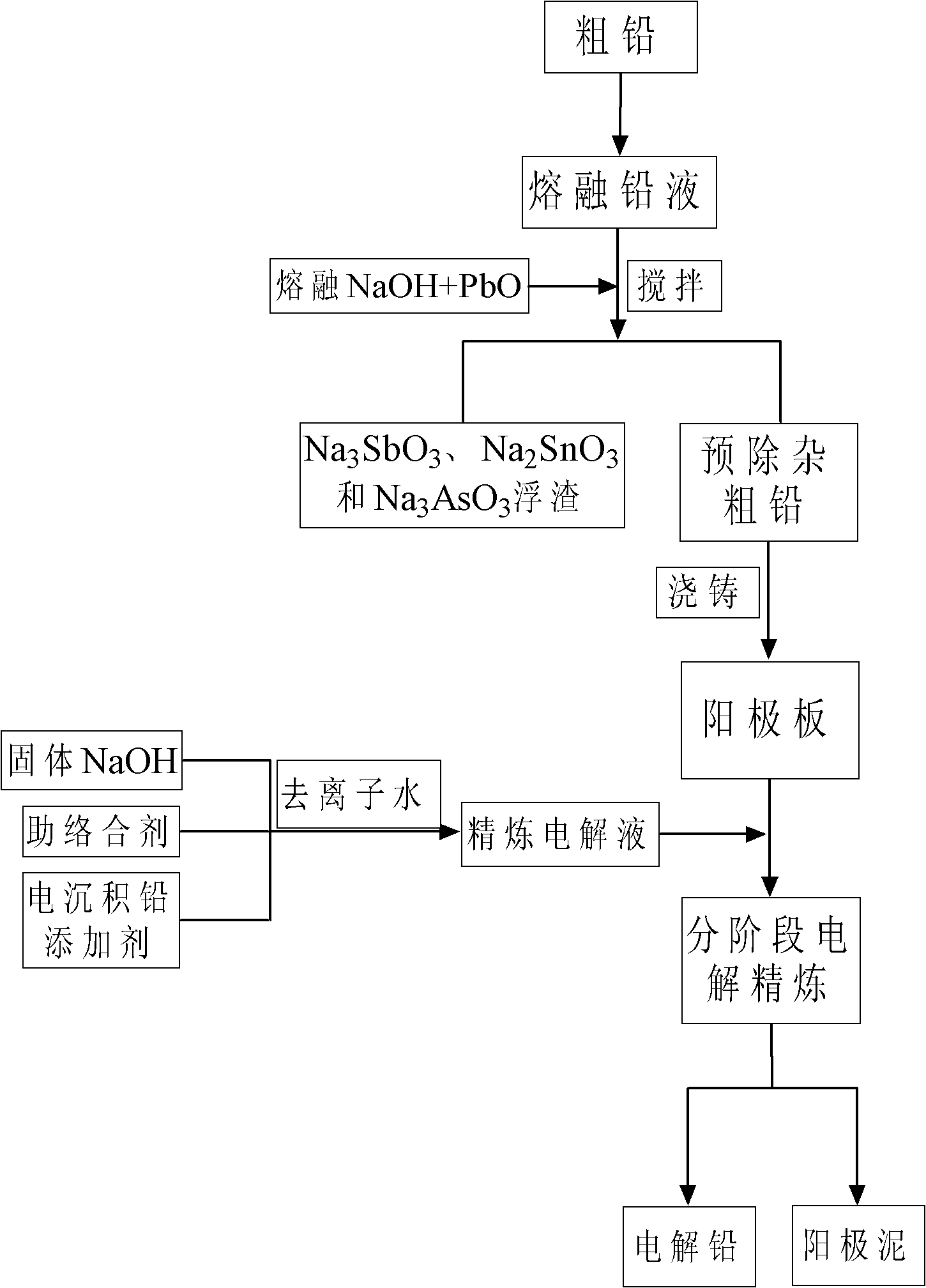
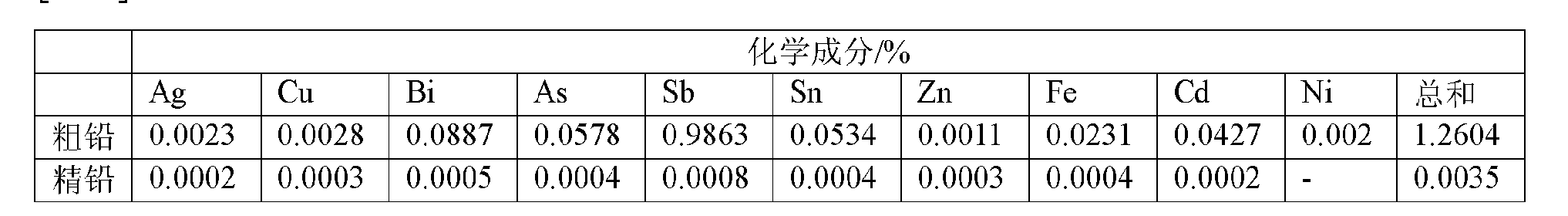
[0076] (1) Get the rough lead of the end decopper that sells on the market, wherein lead content is about 99%. 1 kg of crude lead was first reacted with 100 g of NaOH melt solution with 20 g of PbO at 450 ° C for 45 minutes, so that the impurities such as As, Sb and Sn in it were reacted with the above-mentioned excess PbO and NaOH, and then converted into Na 3 AsO 3 、Na 3 SbO 3 、Na 2 SnO 2 and metallic lead processes;
[0077] (2) The rough lead obtained through the above pre-purification is cast into 8*11*1 (width*height*thick) cm 3 The anode plate with a size of 8*11*0.05 (width*height*thickness) cm 3 The cathode starting plate is made of stainless steel, and the distance between the anode and the cathode is controlled to be 2.6-3.0cm, so that they are placed in the electrolytic cell in parallel;
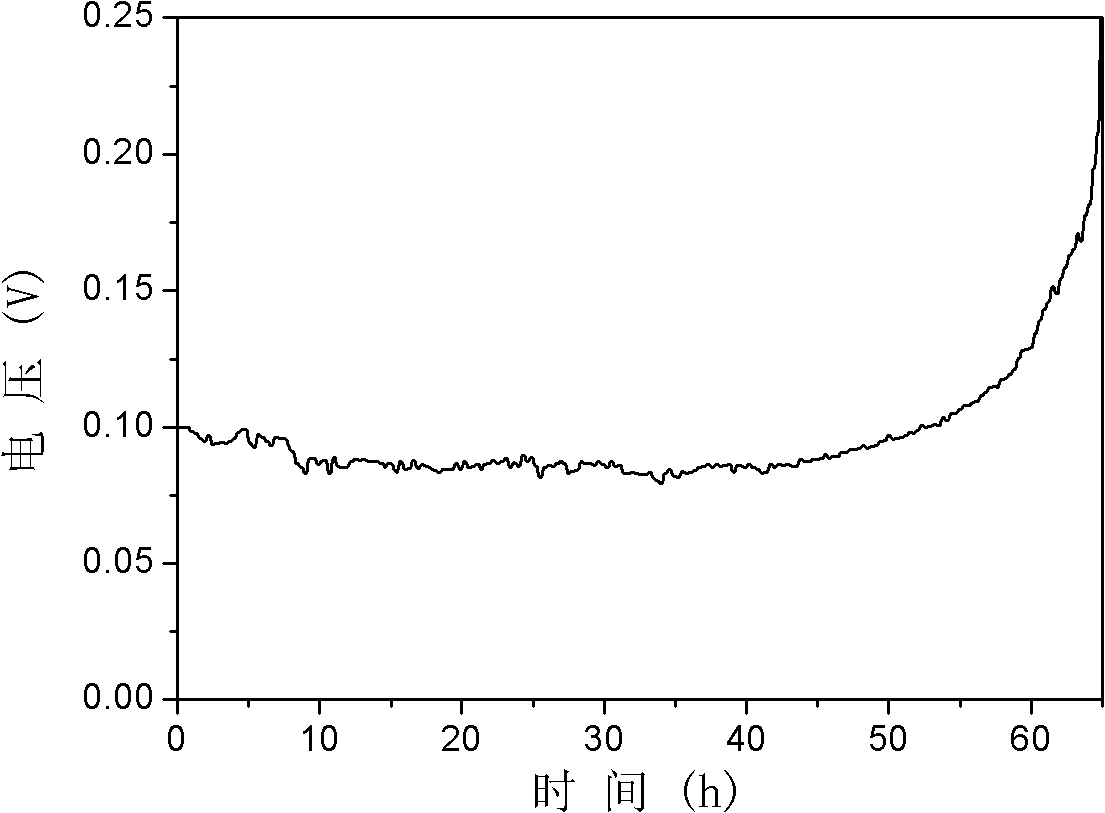
[0078] (3) Configure concentration 300ml to be the PbO mixed solution of 7M NaOH and 0.3M, then add 1g sorbitol to make the concentration of co-complexing agent be 3g / L, s...
Embodiment 2
[0086] (1) Get the common pyrotechnical crude lead on the market, wherein the crude lead content is about 99%. 5kg of crude lead was first stirred and reacted with 400g of NaOH melt solution with 70g of PbO at 450°C for 60 minutes, so that the impurities Sb, Sn and As in the reduced lead reacted with the above-mentioned excess PbO and NaOH, and then converted into Na 3 SbO 3 、Na 2 SnO 2 、Na 3 AsO 3 and metallic lead processes;
[0087] (2) The rough lead obtained through the above pre-purification is cast into 18*20*1.2 (width*height*thick) cm 3 The anode plate with a size of 8*11*0.05 (width*height*thickness) cm 3 The pure lead is used to make the cathode starting plate, so that they are placed in the electrolytic cell in parallel, and the distance between the anode and the cathode is 2.9-3.0cm.
[0088] (3) configuration concentration 1.5L is the PbO mixed solution of 8M NaOH and 0.35M, then add 30g ethylene glycol to make the concentration of co-complexing agent be 2...
Embodiment 3
[0096] (1) Get the reduced lead that common waste lead-acid battery reclaims on the market and obtain, and wherein crude lead content is about 98.7%. 1 kg of crude lead is first stirred and reacted with 100 g of NaOH melt solution with 30 g of PbO at 500 ° C for 45 minutes, so that the main impurities Sb, Sn and As impurities in the reduced lead react with the above-mentioned excess PbO and NaOH, and then convert them into Na 3 SbO 3 、Na 2 SnO 2 、Na 3 AsO 3 and metallic lead processes;
[0097] (2) The rough lead obtained through the above pre-purification is cast into 8*11*1 (width*height*thick) cm 3 The anode plate with a size of 8*11*0.05 (width*height*thickness) cm 3 The cathode starting plate is made of high-quality stainless steel, so that they are placed in the electrolytic cell in parallel, and the distance between the anode and the cathode is 2.5-2.8cm.
[0098] (3) Configure 300ml concentration of PbO mixed solution that is 7.5M NaOH and 0.33M, then add 3.33g ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com