Oral W/O microemulsion preparation of cyclo-trans-4-hydroxyprolyl-L-serine and preparation method of same
A technology of hydoxyserin and milk preparations, which is applied in the field of oral hydoxyserin W/O microemulsion preparations and its preparation, which can solve the problems of difficult to pass through the biofilm barrier, incomplete oral absorption, poor membrane permeability, etc., and achieve stable quality Controllable, bioavailability-enhancing, toxicity-reducing effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
[0021] a. Take 3.706 g of emulsified oil triolein, 1.5625 g of emulsifier Span 80 and 0.3125 g of Tween 80, and 3.751 g of absolute ethanol, and magnetically stir at room temperature to obtain an oil phase;
[0022] b. Weigh 35mg of Hydroxyserin and dissolve it in 1ml of deionized water by ultrasonic to obtain the water phase;
[0023] c. Drop the water phase into the oil phase at room temperature, stir and mix evenly to obtain a clear oily solution, and use a compression method to obtain soft capsules to obtain the oral microemulsion preparation of hydroxyl silk peptide.
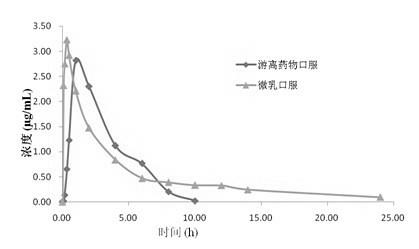
[0024] The average particle size of the obtained microemulsion is 80nm, and the content of hydroxyl serin is 0.33% (W / W).
Embodiment 3
[0026] a. Take 1.849g of emulsified oil triolein, 3.565g of emulsifier monocaprylic / capric glyceride (ODO-L), and 3.565g of absolute ethanol, and stir them evenly with magnetic force at room temperature to obtain the oil phase;
[0027] b. Weigh 40mg of Hydroxyserin, dissolve it in 1.2mL of deionized water by ultrasonic to obtain the aqueous phase;
[0028] c. Drop the water phase into the oil phase at room temperature, stir and mix evenly to obtain a clear oily solution, and use a compression method to obtain soft capsules to obtain the oral microemulsion preparation of hydroxyl silk peptide.
[0029] The average particle size of the obtained microemulsion is 70nm, and the content of hydroxyl serin is 0.39% (W / W).
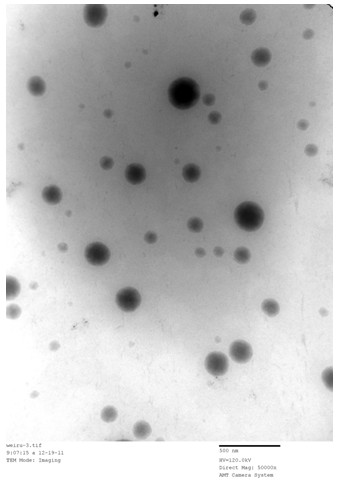
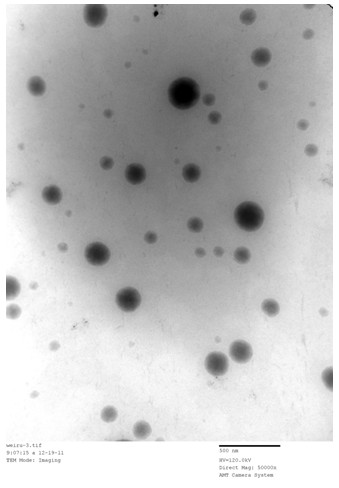
[0030] Observing the oral microemulsion preparation (oily solution with clear appearance) of the embodiment 3 of the present invention with a transmission electron microscope, its transmission electron microscope picture is as follows: figure 1 As shown, the oral...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com