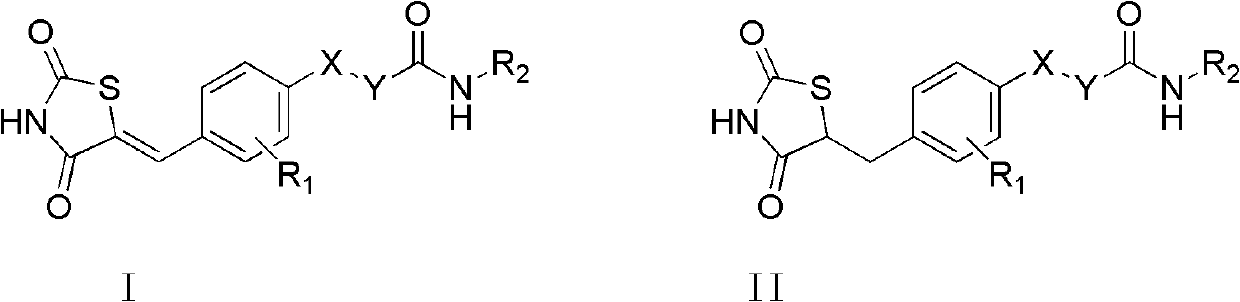
Thiazolidine derivant with GK and PPAR double excitation activity
A technology of thiazolidinedione and derivatives, applied in the field of thiazolidinedione derivatives, can solve the problems of lack of drug safety and toxicity experimental basis, application prospect to be observed and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
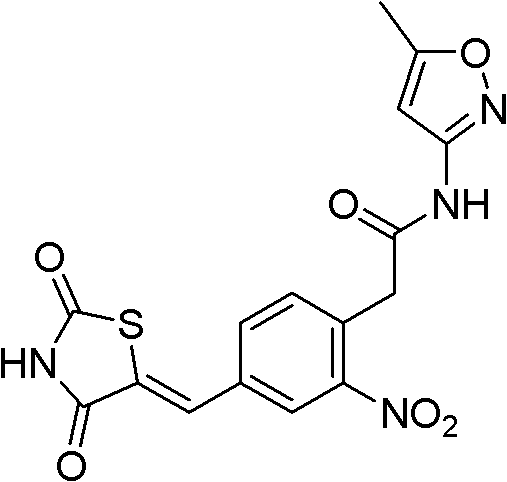
[0066] Example 1: (Z)-2-(4-((2,4-thiazolidinedione-5-ylidene)methyl)-2-nitrophenyl)-N-(5-methylisoxan Azol-3-yl)acetamide
[0067]
[0068] 4-Chloro-3-nitrobenzaldehyde
[0069] Put p-chlorobenzaldehyde (14g, 100mmol) into concentrated sulfur (150mL), add sodium nitrate (8.5g, 100mmol) to the above reaction solution in batches under an ice-water bath, control the reaction temperature not to exceed 40°C, and complete the addition , remove the ice-water bath, and react at room temperature for 24h. After the reaction was completed, the reaction solution was poured into 400 g of crushed ice to obtain a milky white solid product, which was filtered and dried to obtain 16.4 g of a white solid. 1 H NMR (CDCl 3 , 300MHz) δppm: 10.05 (s, 1H, CH), 8.37 (s, 1H, ArH), 8.04 (d, 1H, ArH), 7.76 (d, 1H, ArH); MS (FAB): 185 (M+ 1)
[0070] (Z)-5-(4-Chloro-3-nitrophenylmethylene)thiazolidine-2,4-dione
[0071] Put m-nitro-p-chlorobenzaldehyde (6.2g, 33mmol) and thiazolidinedione (3.9g,...
Embodiment 2
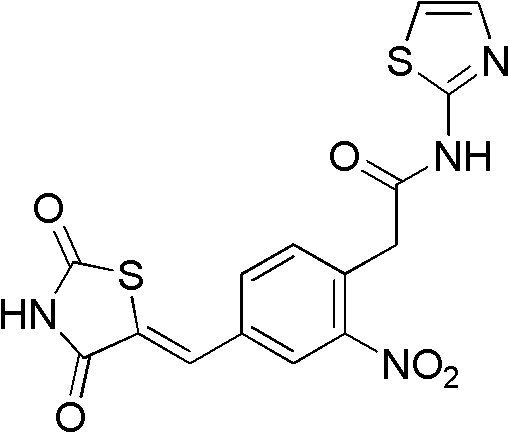
[0078] Example 2: (Z)-2-(4-((2,4-thiazolidinedione-5-ylidene)methyl)-2-nitrophenyl)-N-(thiazol-2-yl) Acetamide
[0079]
[0080] Operation is the same as the synthesis of Example 1. The difference is that the dosage of 2-aminothiazole is (100 mg, 1 mmol). Separation on a silica gel column and recrystallization from methanol gave off-white crystals (44 mg, 11.3%): 1 HNMR (DMSO-d 6 , 300MHz) δppm: 12.65(s, 1H, NH), 9.57(s, 1H, NH), 8.32(s, 1H, CH), 7.89(d, 1H, J=6.9Hz, ArH), 7.85(s, 1H, ArH), 7.81(d, 1H, ArH), 7.71(d, 1H, ArH), 7.65(d, 1H, ArH), 4.04(s, 2H, CH 2 ); HRMS (FAB): C 15 h 11 N 4 o 5 S 2 (M+1), measured value: 391.0170(M+1), calculated value: 391.0157(M+1)
Embodiment 3
[0081] Example 3: (Z)-2-(4-((2,4-thiazolidinedione-5-ylidene)methyl)-2-nitrophenyl)-N-(4-methylthiazole- 2-yl)acetamide
[0082]
[0083] Operation is the same as the synthesis of Example 1. The difference is that the dosage of 2-amino-4-methylthiazole is (114mg, 1mmol). Separation on a silica gel column and recrystallization from methanol gave a light yellow solid (73 mg, 18.1%): 1 H NMR (DMSO-d 6 , 300MHz) δppm: 12.77 (s, 1H, NH), 10.72 (s, 1H, NH), 8.30 (s, 1H, CH), 7.81 (s, 1H, ArH), 7.80 (d, 1H, ArH), 7.65(d, 1H, ArH), 6.22(s, 1H, ArH), 4.22(s, 2H, CH 2 ), 2.23 (s, 3H, CH 3 ); HRMS (FAB): C 16 h 13 N 4 o 5 S 2 (M+1), measured value: 405.0333(M+1), calculated value: 405.03273(M+1)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com