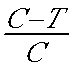Tetrahydroporphin compound and preparation method and application thereof
A technology of tetrahydroporphin and compound, which is applied in the field of tetrahydroporphin compound and its preparation and application, can solve problems such as the listing of tetrahydroporphine PDT drugs, and achieve good application prospects, clear structure and simple preparation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
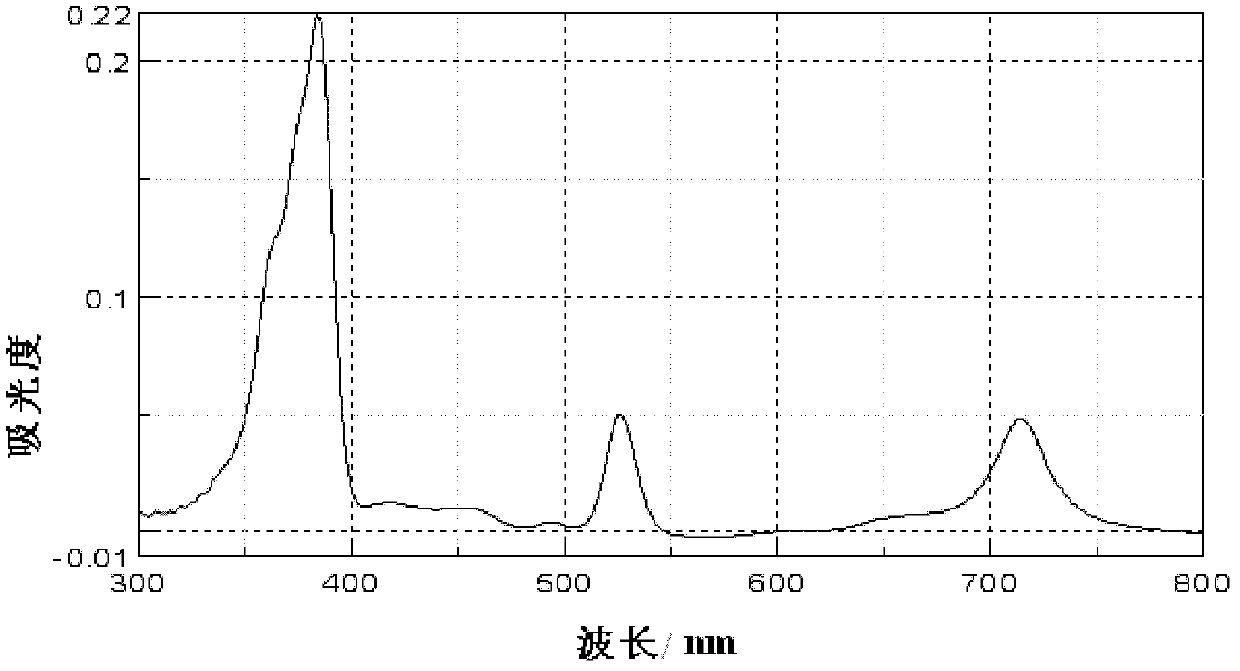
[0037] The preparation method of 2,3-cis-12,13-tetrahydro-2,3,12,13-(tetracarboxymethyl)-5,10,15,20-tetraphenylporphin specifically comprises the following steps:
[0038] (1) Synthesis of 2,3-dihydro-2,3-bis(dicyanomethyl)-5,10,15,20-tetraphenylporphine
[0039] In a 500mL three-neck flask, add 3.38g (24.4mmol) K 2 CO 3and 2.86mL (30.4mmol) of malononitrile, under the protection of nitrogen, inject 100mL of freshly distilled tetrahydrofuran with a syringe, and stir and reflux for 1h. Then 2g (3.04mmol) of 2-nitro-5,10,15,20-tetraphenylporphin was added to the mixture, the mixture was continued to reflux and stirred, and the reaction was monitored by TLC. After 48 hours, the reaction was almost complete. Add 2L dichloromethane to the reaction solution for extraction, wash with water 3 times, and wash with saturated NaCl for 3 times, then anhydrous NaCl 2 SO 4 Dry and concentrate. The crude product was separated by column chromatography, using 300-400 mesh silica gel as th...
Embodiment 2
[0049] Photodynamic Antiproliferation Experiment of Photosensitizers on Colon Cancer SW480 Cells
[0050] Tested cells: colon cancer cells SW480
[0051] Test drug: 2,3-cis-12,13-tetrahydro-2,3,12,13-(tetracarboxymethyl)-5,10,15,20-tetraphenylporphine (hereinafter referred to as photosensitizer 1), hematoporphyrin derivative HpD (produced by Beijing Institute of Pharmaceutical Industry).
[0052] Light source: MTZ-1 pulse laser cancer treatment machine; SD2490 laser power measuring instrument.
[0053] Photodynamic anti-tumor cell proliferation experiment: cells in the logarithmic growth phase were digested with trypsin, and the complete medium was resuspended into a cell suspension, which was then inoculated in a 96-well plate, 100 μl per well, placed at 37 ℃5%CO 2 Cultivate in an incubator, add five different photosensitizers at the same concentration after 24 hours; replace with fresh medium after 48 hours, and then carry out light (XD-635AB photodynamic PDT laser therap...
Embodiment 3
[0057] photosensitizer on mouse S 180 Photodynamic therapy experiment of sarcoma
[0058] Test animals: Outbred Kunming strain mice with an average weight of 18-24 g, S180 sarcoma mice (provided by the Institute of Materia Medica, Chinese Academy of Sciences)
[0059] Test drug: 2,3-cis-12,13-tetrahydro-2,3,12,13-(tetracarboxymethyl)-5,10,15,20-tetraphenylporphine (hereinafter referred to as photosensitizer 1) Under aseptic conditions, the above-mentioned medicine was dissolved in a minimum amount of Tween-80 in normal saline and diluted to a 0.5mg / mL solution for later use, hematoporphyrin derivative HpD (produced by Beijing Pharmaceutical Industry Research Institute).
[0060] Light source: MTZ-1 pulse laser cancer treatment machine; SD2490 laser power measuring instrument.
[0061] Photodynamic injury experiment of mouse S180 sarcoma: S180 sarcoma was inoculated subcutaneously in the anterior chest of mice under aseptic conditions. Litters of the same sex were randomly d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com