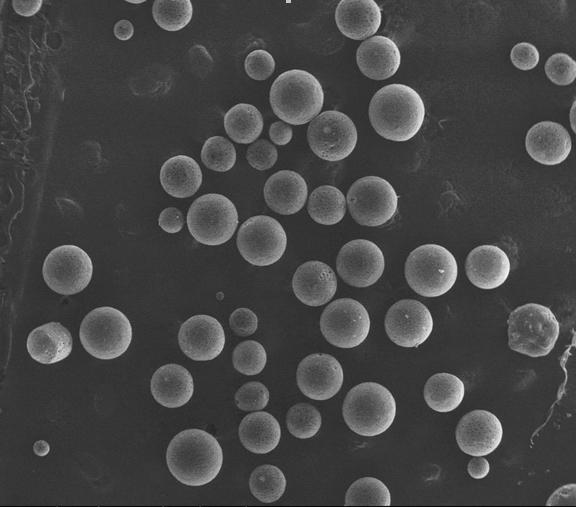
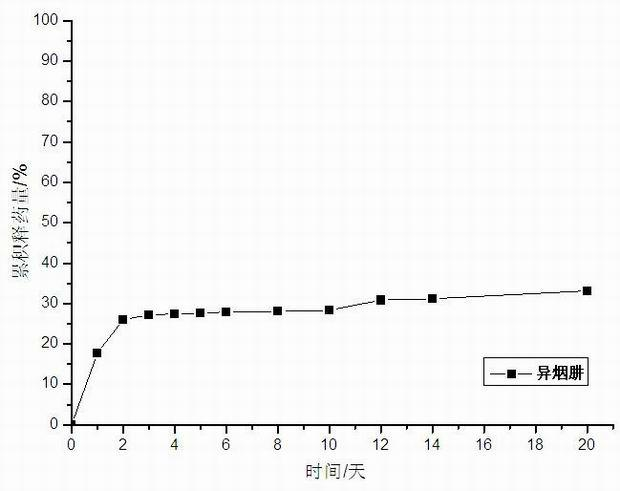
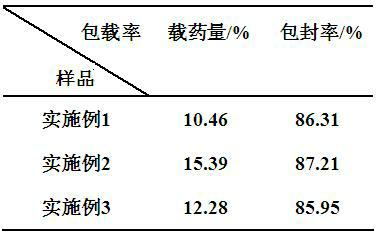
Method for preparing medicine-carrying hydroxyapatite/poly glycolide-co-lactide (PLGA)/chitosan demixing microspheres
A technology for drug hydroxyapatite and hydroxyapatite is applied in the field of preparation of biomedical materials, and can solve the problems of low drug concentration, insufficient therapeutic effect, insufficient effective drug content, irregular spherical structure, etc. release effect, improved drug encapsulation efficiency, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] (1) Dissolve 0.2g of isoniazid in 10mL of deionized water, add 1.0g of hydroxyapatite powder, stir at a speed of 200r / min for 15 minutes in the dark to obtain a translucent emulsion, and then freeze-dry for 54 hours get powder;
[0030] (2) Dissolve 0.5g PLGA in 10mL dichloromethane to obtain 5.0% (wt.) PLGA solution; mix 10mL PLGA solution and 1.5g powder evenly to obtain 10mL HA / PLGA blend containing isoniazid;
[0031] (3) Heat 100mL of deionized water to 90°C, dissolve 2g of polyvinyl alcohol 1788 in it, cool down to 40°C, and obtain 2.0% (wt.) polyvinyl alcohol aqueous solution; dissolve 0.4g of chitosan in 10mL of 1 % (wt.) acetic acid aqueous solution to obtain 40% (wt.) chitosan solution; mix 10mL chitosan solution with 100mL polyvinyl alcohol aqueous solution to obtain 110mL chitosan / polyvinyl alcohol solution;
[0032] (4) Add 10mL of the isoniazid-containing hydroxyapatite / PLGA blend obtained in step (2) into the 110mL chitosan / polyvinyl alcohol solution obt...
Embodiment 2
[0036] (1) Dissolve 1.0g of isoniazid in 10mL of deionized water, add 1.0g of hydroxyapatite powder, stir at a speed of 100r / min for 20 minutes in the dark to obtain a translucent emulsion, and then freeze-dry for 72h get powder;
[0037] (2) Dissolve 1.5g PLGA in 10mL dichloromethane to obtain 15% (wt.) PLGA solution; mix 10mL PLGA solution and 2.0g powder evenly to obtain 10mL HA / PLGA blend containing isoniazid;
[0038] (3) Heat 200mL of deionized water to 100°C, dissolve 2g of polyvinyl alcohol 1788 in it, cool down to 45°C, and obtain 1.0% (wt.) polyvinyl alcohol aqueous solution; dissolve 0.6g of chitosan in 10mL of 3 % (wt.) acetic acid aqueous solution to obtain 6.0% (wt.) chitosan solution; mix 10mL chitosan solution with 200mL polyvinyl alcohol aqueous solution to obtain 210mL chitosan / polyvinyl alcohol solution;
[0039] (4) Add 10mL of the isoniazid-containing hydroxyapatite / PLGA blend obtained in step (2) into the 210mL chitosan / polyvinyl alcohol solution obtaine...
Embodiment 3
[0041] (1) Dissolve 0.3g of isoniazid in 10mL of deionized water, add 0.6g of hydroxyapatite powder, stir at a speed of 160r / min for 16 minutes in the dark to obtain a translucent emulsion, and then freeze-dry for 36 hours get powder;
[0042] (2) Dissolve 0.8g PLGA in 10mL dichloromethane to obtain 8.0% (wt.) PLGA solution; mix 10mL PLGA solution and 0.9g powder evenly to obtain 10mL HA / PLGA blend containing isoniazid;
[0043] (3) Heat 150mL deionized water to 95°C, dissolve 2.25g polyvinyl alcohol 1788 in it, cool down to 40°C, and obtain 1.5% (wt.) polyvinyl alcohol aqueous solution; dissolve 0.5g chitosan in 10mL 5.0% (wt.) chitosan solution was obtained in 2% (wt.) acetic acid aqueous solution; 10mL chitosan solution was mixed with 150mL polyvinyl alcohol aqueous solution to obtain 160mL chitosan / polyvinyl alcohol solution;
[0044] (4) Add 10mL of the isoniazid-containing hydroxyapatite / PLGA blend obtained in step (2) into the 160mL chitosan / polyvinyl alcohol solution ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com