Method for treating waste liquid produced during on-line chemical oxygen demand (COD) determination with potassium dichromate method
A potassium dichromate method and waste liquid treatment technology, applied in chemical instruments and methods, water/sewage multi-stage treatment, water/sludge/sewage treatment, etc., to achieve fast reaction speed, simple operation, and removal of toxic heavy metals Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
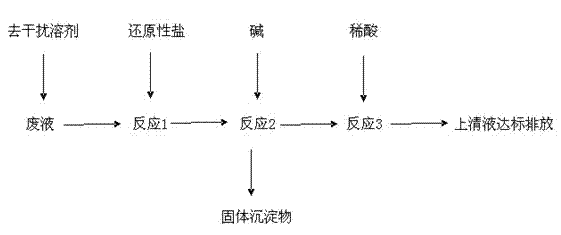
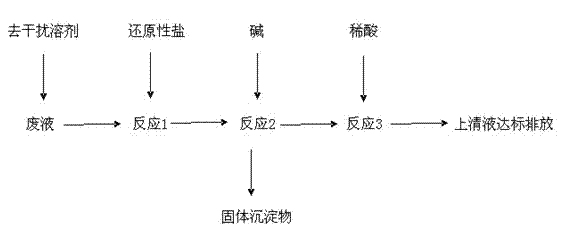
[0029] Add 1L of waste liquid into the reactor, the concentration of metal ions in the waste liquid is, Hg 2+ : 1g / L, Cr 3+ : 2.83g / L, Ag + : 0.1g / L; prepare interference-removing solvent: weigh potassium carbonate and sodium bicarbonate, prepare K + 、Na + 、HCO 3 - , CO 3 2- 5000g of ion solution, the concentration of each ion is: K + : 0.2g / L, Na + : 0.11g / L, HCO 3 - : 0.29g / L, CO 3 2- : 0.15g / L, the pH range of the solution is between 6.5 and 8.5; add 5000g of the prepared interference-removing solvent to the waste liquid, and then add 0g of reducing salt to it, at this time, the waste liquid does not contain Cr 6+ ions, containing only Cr 3+ Ion, stirring reaction, about 2 minutes to complete the reaction;
[0030] Add 10g alkali again in the reactor, the kind of alkali is NaOH or Na 2 CO 3 Any one of them, after stirring the reaction, the reaction ends in about 3 minutes;
[0031] Stand still, drain the supernatant, add 10% dilute hydrochloric acid to the ...
Embodiment 2
[0033] Add 1L of waste liquid into the reactor, the concentration of metal ions in the waste liquid is, Hg 2+ : 6.8g / L, Cr 6+ : 2.83g / L, Cr 3+ : 0g / L, Ag + : 2.22g / L; Preparation of interference-removing solvent: Weigh potassium carbonate and sodium bicarbonate, prepare K + 、Na + 、HCO 3 - , CO 3 2- Ionic solution 1000g, the concentration of each ion is: K + : 0.28g / L, Na + : 0.15g / L, HCO 3 - : 0.40g / L, CO 3 2- : 0.22g / L, the pH range of the solution is between 6.5 and 8.5; add 1000g of the prepared interference-removing solvent to the waste liquid, and then add 60g of reducing salt to it, and the reducing salt is Na 2 SO 3 30g, Na 2 S 2 o 3 ·5H 2 030g stirring reaction, about 2 minutes reaction finishes;
[0034] Add 480g alkali again in the reactor, the kind of alkali is NaOH or Na 2 CO 3 Any one of them, after stirring the reaction, the reaction ends in about 3 minutes;
[0035] Put it still, drain the supernatant, add hydrochloric acid with a concentra...
Embodiment 3
[0037] Add 1L of waste liquid into the reactor, the concentration of metal ions in the waste liquid is, Hg 2+ : 2.8g / L, Cr 6+ : 1.83g / L, Cr 3+ : 1.0g / L, Ag + : 1.22g / L; Preparation of interference-removing solvent: Weigh potassium carbonate and sodium bicarbonate, prepare K + 、Na + 、HCO 3 - , CO 3 2- 3000g of ion solution, the concentration of each ion is: K + : 0.33g / L, Na + : 0.19g / L, HCO 3 - : 0.50g / L, CO 3 2- : 0.25g / L, the pH range of the solution is between 6.5 and 8.5; add 3000g of the prepared interference-removing solvent to the waste liquid, and then add 100g of reducing salt, the reducing salt FeSO 4 ·7H 2 O is 20g, Fe 2 (SO 3 ) 3 20g, FeSO 4 30g, FeCl 2 For 30g stirring reaction, the reaction ends in about 2 minutes;
[0038] Add 480g alkali again in the reactor, the kind of alkali is NaOH or Na 2 CO 3 Any one of them, after stirring the reaction, the reaction ends in about 3 minutes;
[0039] Stand still, drain the supernatant, add hydrochlo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com