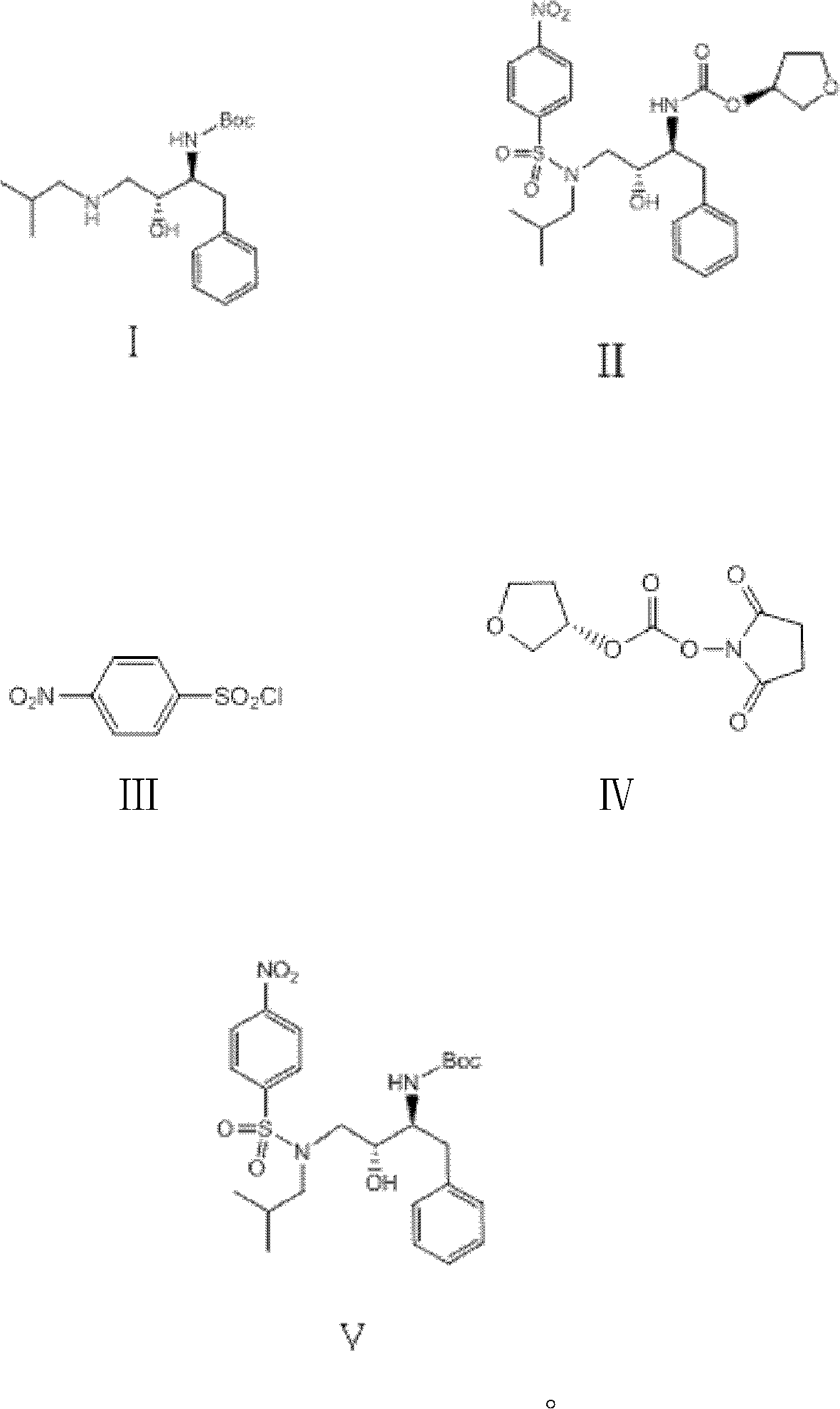
Synthesizing method of antiviral drug amprenavir intermediate
An antiviral drug and a synthesis method technology, applied in the direction of organic chemistry and the like, can solve the problems of cumbersome operation, difficult to reuse, large amount of organic solvent, etc., to reduce the use of organic solvents, reduce post-processing operations, and achieve high product yield. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
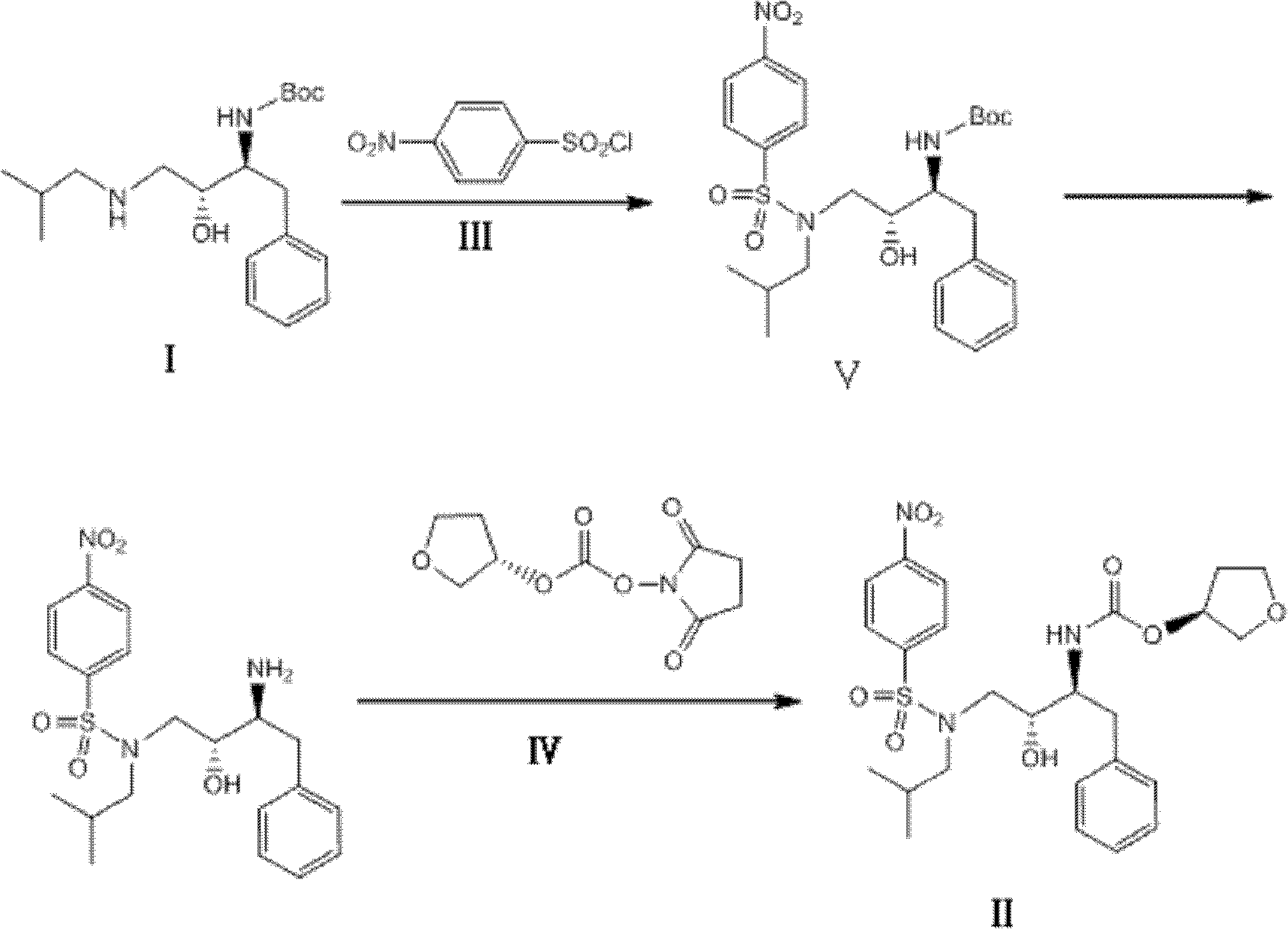
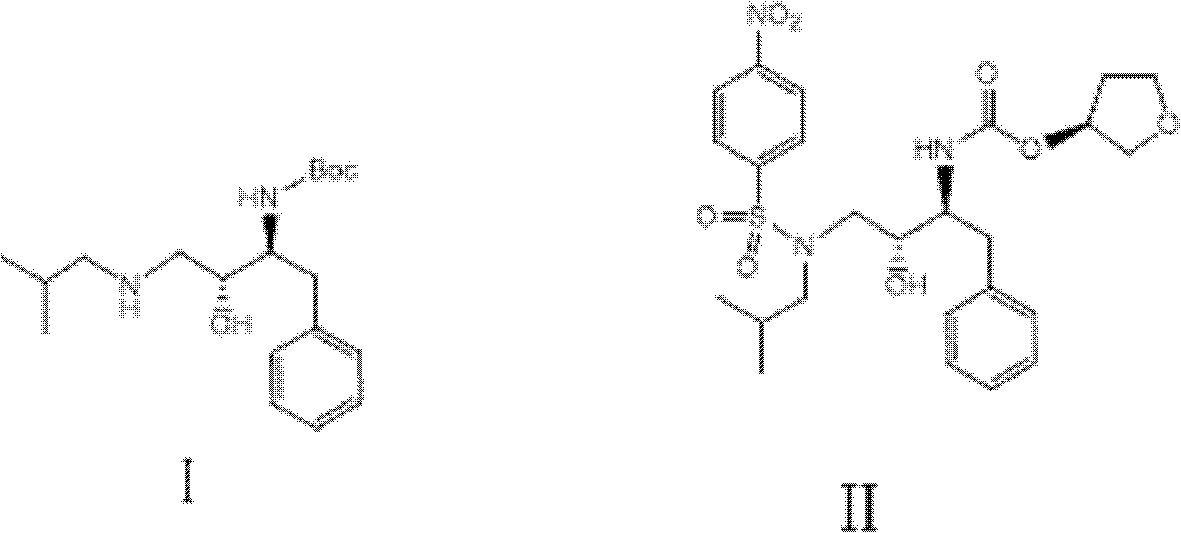
[0024] The raw material (2S, 3R)-3-hydroxyl-4-(isobutylamino)-1-phenyl-2-butylcarbamate tert-butyl ester (I) (34.0g, 0.1mol) and triethylamine (20.5g , 0.2mol) was dissolved in dichloromethane (150mL), and a dichloromethane solution (150mL) of 4-nitrobenzenesulfonyl chloride (27.0g, 0.12mol) was added dropwise at -10°C, and the reaction was stirred at 30°C for 20h Afterwards, the liquid chromatography analysis formula (I) peak disappears, cools down to 10 ℃, feeds hydrogen chloride gas (1mol) reaction within 6h, adds the sodium hydroxide aqueous solution 300mL alkali washing of 2mol / L three times, stands for stratification, takes the organic Layer was dried with anhydrous sodium sulfate (30g), and a dichloromethane solution (100mL) of (S)-3-tetrahydrofuryloxy-succinimidyl carbonate (23.0g, 0.1mol) was added dropwise at 20°C , After reacting for 20h, dichloromethane was removed under reduced pressure, washed with water and dried to obtain the described amprenavir intermediate (...
Embodiment 2
[0026]The starting material (2S, 3R)-3-hydroxyl-4-(isobutylamino)-1-phenyl-2-butylcarbamate tert-butyl ester (I) (34.0g, 0.1mol) and triethylamine (30.8g , 0.3mol) was dissolved in toluene (150mL), and a toluene solution (150mL) of 4-nitrobenzenesulfonyl chloride (33.8g, 0.15mol) was added dropwise at 10°C, stirred and reacted at 45°C for 30h, and the liquid chromatographic analysis formula (1) The peak disappears, and at this temperature, the flow rate in 20h feeds hydrogen chloride gas (2mol) to react, and the gained reaction solution adds 2mol / L potassium hydroxide aqueous solution 300mL alkali washes three times, leaves standstill and divides the water layer, and the organic layer is used Anhydrous magnesium sulfate (35g) was dried, and a toluene solution (100mL) of (S)-3-tetrahydrofuryloxy-succinimidyl carbonate (16.1g, 0.7mol) was added dropwise at 50°C, and after 20h of reaction, the Remove the toluene under pressure, wash and dry to obtain the described amprenavir inte...
Embodiment 3
[0028] Starting material (2S, 3R)-3-hydroxyl-4-(isobutylamino)-1-phenyl-2-butylcarbamate tert-butyl ester (I) (34.0g, 0.1mol) and pyridine (24g, 0.3mol ) was dissolved in dichloroethane (150mL), and 4-nitrobenzenesulfonyl chloride (27.0g, 0.12mol) in dichloroethane solution (150mL) was added dropwise at -10°C, stirred at 40°C for 20h, and the liquid phase The peak of chromatographic analysis formula (I) disappears, lower the temperature to -10°C, feed hydrogen chloride gas (1.2mol) to react within 6h, add 0.5mol / L sodium carbonate aqueous solution 1000mL alkali wash three times, let stand to separate the water layer, organic layer Dry with anhydrous calcium chloride (26g), add (S)-3-tetrahydrofuryloxy-succinimidyl carbonate (46.0g, 0.2mol) in dichloroethane solution (100mL) dropwise at 10°C , After reacting for 40h, dichloroethane was removed under reduced pressure, washed with water and dried to obtain the amprenavir intermediate (5) (3S)-3-tetrahydrofuryloxy N-[(1S, 2R)-3-( ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com