Salt of carbostyril formic acid compound as well as preparation method and application of salt
A methoxyquinolone formic acid and compound technology, which is applied to the salt of 8-methoxyquinolone formic acid compounds, the application field of moxifloxacin hydrochloride quality detection and analysis, can solve the problem of affecting the quality of moxifloxacin hydrochloride and increase clinical Drug risks, undisclosed determination of moxifloxacin, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Example 1 Mode Synthesis of the hydrochloride salt of the compound
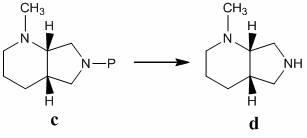
[0048] (1), N-methyl-(S,S)-2,8-diazabicyclo[4,3,0]nonane ( d )Synthesis
[0049]
[0050] Measure 30g formula a The compound was dissolved in 200ml of dry dichloromethane, and 46.5g of di-tert-butyl dicarbonate (Boc 2 O) A solution in 50 ml of dichloromethane. Stir at room temperature for 3 h, concentrate under reduced pressure to remove the solvent, and use dichloromethane-methanol (20:1) as the eluent to separate the residue by silica gel column chromatography, collect the product, concentrate under reduced pressure to remove the solvent, and add the residue to Dissolve 100ml of dichloromethane, add dropwise a solution of 35g of iodomethane dissolved in 50ml of dichloromethane at room temperature, stir at room temperature for 2 hours, concentrate under reduced pressure and evaporate the solvent, add 50ml of methanol to the residue to dissolve, add dropwise methanolic hydrochloric acid ...
Embodiment 2
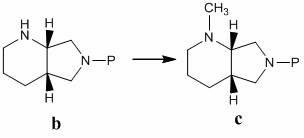
[0057] Example 2 Mode Synthesis of the sodium salt of the compound
[0058] (1), N-methyl-(S,S)-2,8-diazabicyclo[4,3,0]nonane ( d )Synthesis
[0059] Measure 10g formula a The compound was dissolved in 100ml of dry dichloromethane, and 15.5g of di-tert-butyl dicarbonate (Boc 2 O) A solution in 15 ml of dichloromethane. Stir at room temperature for 5 hours, concentrate under reduced pressure to remove the solvent, and use dichloromethane-methanol (20:1) as the eluent to separate the residue by silica gel column chromatography, collect the product, concentrate under reduced pressure to remove the solvent, and add the residue to Dissolve 35ml of dichloromethane, add dropwise a solution of 8.8g dimethyl sulfate dissolved in 15ml of dichloromethane at room temperature, stir at room temperature for 3h, concentrate under reduced pressure and evaporate the solvent, add 20ml methanol to the residue to dissolve, add dropwise methanol hydrochloric acid solution (2ml Conce...
Embodiment 3
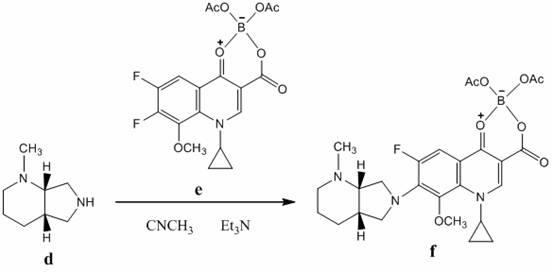
[0064] Example 3 Mode mesylate of compound
[0065] (1), N-methyl-(S,S)-2,8-diazabicyclo[4,3,0]nonane ( d )Synthesis
[0066] Measure 10g formula a The compound was dissolved in 100ml of dry dichloromethane, and 15.5g of di-tert-butyl dicarbonate (Boc 2 O) A solution in 15 ml of dichloromethane. Stir at room temperature for 5 hours, concentrate under reduced pressure to remove the solvent, and use dichloromethane-methanol (20:1) as the eluent to separate the residue by silica gel column chromatography, collect the product, concentrate under reduced pressure to remove the solvent, and add the residue to Dissolve 35ml of dichloromethane, add dropwise a solution of 6.3g dimethyl carbonate dissolved in 15ml of dichloromethane at room temperature, stir at room temperature for 5h, concentrate under reduced pressure and evaporate the solvent, add 20ml of methanol to the residue to dissolve, add dropwise methanolic hydrochloric acid solution (2ml Dissolve concentrated ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com