Method for producing less-barium fine strontium salts from celestite
A production method and lapis lazuli technology, applied in the direction of calcium/strontium/barium nitrate, calcium carbonate/strontium/barium, calcium/strontium/barium halide, etc., can solve the problem of high cost of raw materials, increased consumption of strontium carbonate, approval, Problems such as transportation and storage troubles, to achieve the effect of low cost of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
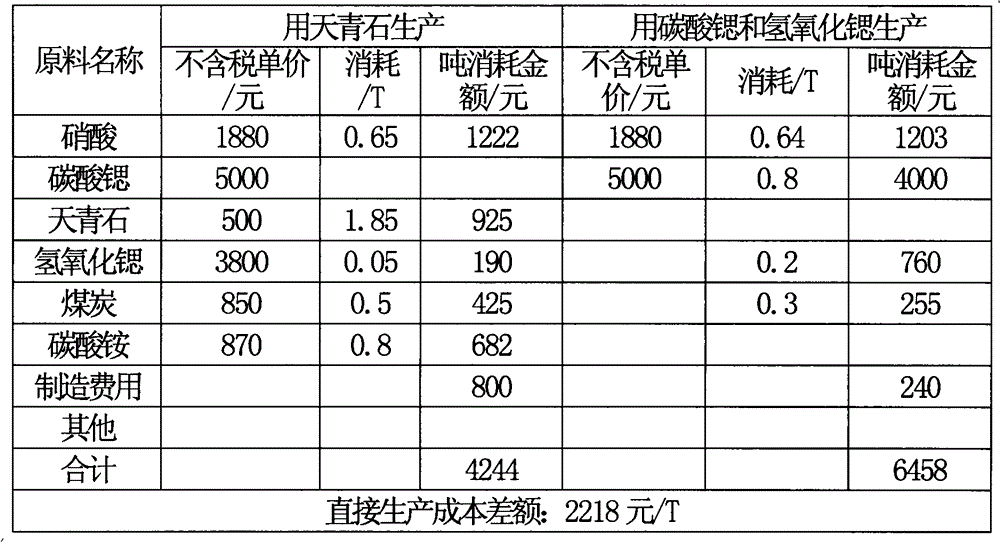
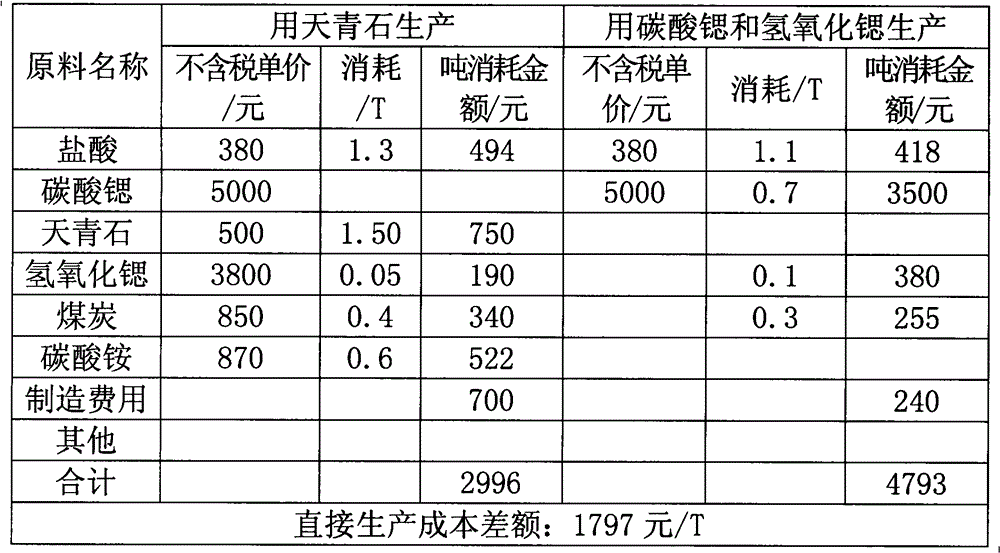
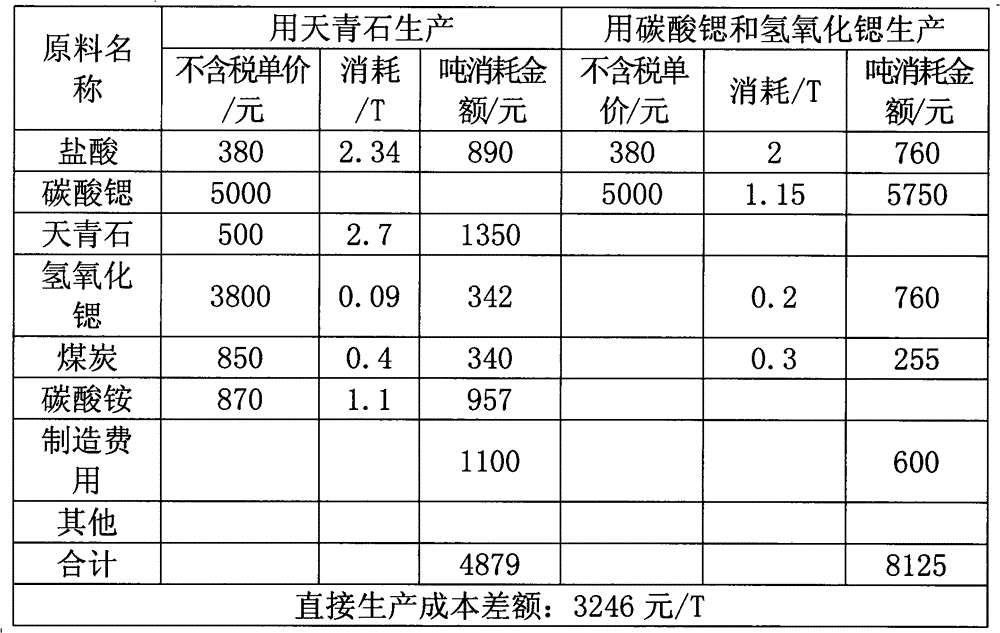
Image
Examples
Embodiment 1
[0046] a, refinement: crush the lapis lazuli and then grind it to pass through a 200-mesh sieve;
[0047]b. Pickling: react with dilute hydrochloric acid with a concentration of 12% and ground lapis lazuli;
[0048] c. Metathesis: add refined lapis lazuli to 10% ammonium carbonate aqueous solution and stir evenly; then under airtight conditions, the reaction pressure is 0.2Mpa, the reaction material temperature is 110±2°C, and the reaction is taken after 5 hours , when the strontium carbonate content of the solid part in the material is 81%, stop heating, after filtering, the gained solid is washed with clear water to obtain the strontium carbonate crude product;
[0049] e: react the strontium carbonate crude product with nitric acid, control the pH value of the reaction end point to be 3, and obtain a strontium nitrate solution with a concentration of 30Be;
[0050] f: Add 3 liters / cubic hydrogen peroxide and 2 kg / cubic activated carbon to the strontium nitrate solution, ad...
Embodiment 2
[0055] a, refinement: crush the lapis lazuli and then grind it to pass through a 200-mesh sieve;
[0056] b. Pickling: react with dilute hydrochloric acid with a concentration of 15% and ground lapis lazuli;
[0057] c. Metathesis: add refined lapis lazuli to 10% ammonium carbonate aqueous solution, and stir evenly; then under airtight conditions, the reaction pressure is 0.26Mpa, the reaction material temperature is 110±2°C, and the reaction is taken after 5 hours , when the strontium carbonate content of the solid part in the material is 78%, stop heating, after filtering, the gained solid is washed to obtain the strontium carbonate crude product;
[0058] e: react the strontium carbonate crude product with nitric acid, control the pH value of the reaction end point to 4.5, and obtain a strontium nitrate solution with a concentration of 35Be;
[0059] f: Add 2 liters / cubic hydrogen peroxide and 1 kg / cubic activated carbon to the strontium nitrate solution, add strontium hyd...
Embodiment 3
[0065] a, refinement: crush the lapis lazuli and then grind it to pass through a 200-mesh sieve;
[0066] b. Pickling: react with dilute hydrochloric acid with a concentration of 10% and ground lapis lazuli;
[0067] c. Metathesis: add refined lapis lazuli to 10% ammonium carbonate aqueous solution, and stir evenly; then under airtight conditions, the reaction pressure is 0.3Mpa, the reaction material temperature is 115±2°C, and the reaction is taken after 5 hours , when the strontium carbonate content of the solid part in the material is 76%, stop heating, after filtering, the gained solid is washed to obtain the strontium carbonate crude product;
[0068] j: reacting the strontium carbonate crude product with hydrochloric acid, controlling the pH value at the end of the reaction to be 4, and obtaining a strontium chloride solution with a concentration of 30Be;
[0069] k: Add 1 liter / cubic hydrogen peroxide with a concentration of 27% and 1.5 kg / cubic activated carbon to th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com