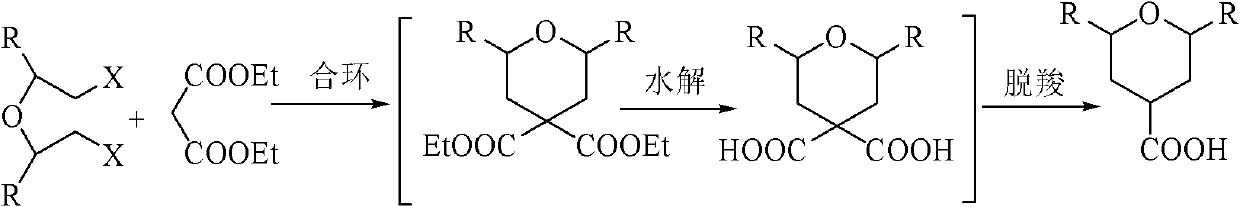
Preparation method of tetrahydropytanyl-4-formic acid and derivatives thereof by one-pot process
A technology of tetrahydropyran and derivatives, applied in organic chemistry and other fields, can solve the problems of expensive raw materials, complicated operation, cumbersome operation, etc., and achieve the effect of stable process, simple operation and improved overall yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1: A kind of one-pot method prepares tetrahydropyran-4-carboxylic acid The method is characterized in that the specific preparation steps are as follows:
[0028] (1) Ring closure: Add 417.6kg toluene (1g / 6mL), 172.5kg potassium carbonate (2.5eq) and 19.3kg tetrabutylammonium bromide (0.12eq) to a 1000L reactor in sequence, and heat up to 55±2°C , drop by 80.0kg diethyl malonate (1eq) and 93.0kg dichloroethyl ether (1:1.3eq) mixed solution, after dropping, keep warm at 50±2°C for 15h; after the reaction, cool the system down to 20-30°C, add 480kg of water (1g / 6mL) and 278.4kg of toluene (1g / 4mL) Extraction and liquid separation to obtain an organic phase;
[0029](2) Hydrolysis: Add 480kg (1g / 5mL) of 50% potassium hydroxide solution to the organic phase obtained in step (1), raise the temperature of the system to 80±2°C, keep it warm for 30h, and after the reaction is complete, cool the system to 20-30°C , add 160kg of water (1g / 2mL), separate the liquid...
Embodiment 2
[0031] Embodiment 2: A kind of one-pot method prepares 2,6-dimethyl-4-formic acid pyran The method is characterized in that the specific preparation steps are as follows:
[0032] (1) Ring closure: Add 277.4kg of ethylbenzene (1g / 1mL) to a 3000L reactor, start stirring, then add 424.0kg of anhydrous sodium carbonate (1:2.0eq) and 73.9kg of tetrabutylammonium iodide ( 1:0.05eq), warming up to 63±2°C; dropwise adding 320.0kg diethyl malonate (1eq) and 843.1kg 2 dibromoisopropyl ether (1:1.0eq) mixed solution, after dropping, keep warm at 63±2°C for 3h, after the reaction is complete, cool the system down to 20-30°C, add the reaction system to 320kg water (1g / 1mL) and 277.4kg ethylbenzene (1g / 1mL) extraction, liquid separation to obtain an organic phase;
[0033] (2) Hydrolysis: Add 768kg (1g / 2mL) of prepared 50% sodium hydroxide solution to the organic phase system obtained in step (1), heat up the system to 85±5°C, keep it warm at 85±5°C for 10h, and the reaction is complet...
Embodiment 3
[0035] Embodiment 3: A kind of one-pot method prepares 2,6-dinitro-4-formic acid pyran The method is characterized in that the specific preparation steps are as follows:
[0036] (1) Ring closure: Add 1290kg xylene (1g / 10mL) to a 5000L reactor, start stirring, then add 414.0kg anhydrous potassium carbonate (1:3.0eq) and 52.2kg tetrabutylammonium chloride (1 : 0.2eq), the temperature was raised to 48±2°C; 150.0kg diethyl malonate (1eq) and 781.9kg 1,1'-dinitro-2,2'-diiodoethyl ether were added dropwise (1:2.0eq) mixed solution, after dropping, keep warm at 48±2°C for 20h, after the reaction is complete, cool the system down to 20-30°C, add the reaction system to 1500kg water (1g / 10mL) and 1290kg xylene ( 1g / 10mL) extraction, liquid separation to obtain an organic phase;
[0037] (2) Hydrolysis: Add 1800kg (1g / 10mL) of prepared 50% potassium hydroxide solution to the organic phase system obtained in step (1), raise the temperature of the system to 70±5°C, keep it at 70±5°C f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com