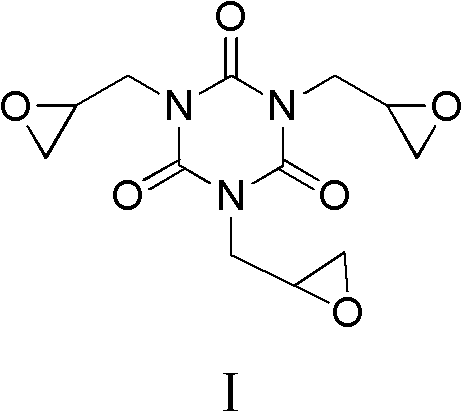
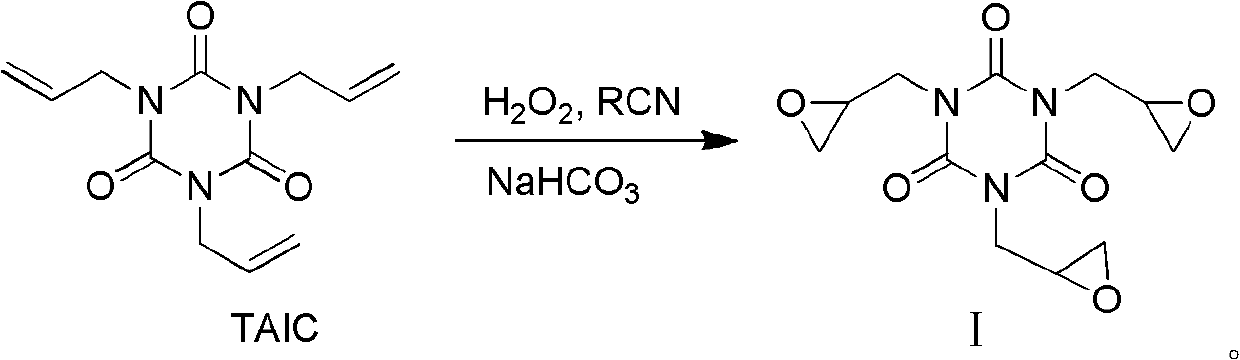
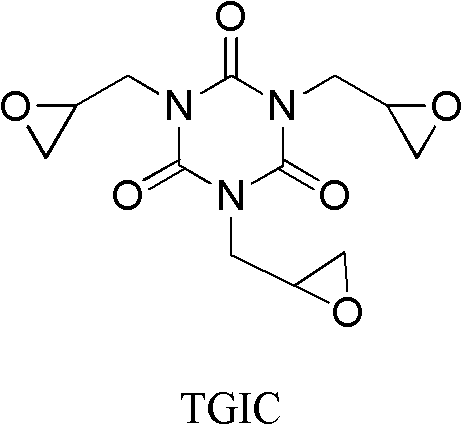
Preparation method of triglycidyl isocyanurate
A technology of triglycidyl ester and cyanuric acid, applied in the direction of organic chemistry, can solve the problem of incomplete epoxidation of double bonds, and achieve the effect of less equipment corrosion, light color and high quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Preparation of triglycidyl cyanurate
[0030]
[0031] Methanol (50ml), ammonium bicarbonate (0.07mol), triallyl isocyanurate (0.03mol), acrylonitrile (0.09mol) were stirred for 15 minutes, and 30% hydrogen peroxide (0.1mol) was added at 25 Stir and react at ~60°C for 2 hours, add 5 g of 30% hydrogen peroxide, continue the reaction for 6 to 8 hours, and complete the reaction. The reaction solution was post-treated to obtain triglycidyl cyanurate with a yield of 80.1% and a melting point of 103-115°C; 1 H NMR (400MHz, CDCl 3 )δ: 2.70~2.86(m, 6H, 3×CH 2 ), 3.27~3.28(m, 3H, 3×CH), 4.07~4.16(m, 6H, 3×CH 2 ).
Embodiment 2
[0033] Preparation of triglycidyl cyanurate
[0034] Ethanol (50ml), potassium bicarbonate (0.05mol), triallyl isocyanurate (0.03mol), and acetonitrile (0.09mol) were stirred for 15 minutes, and 30% hydrogen peroxide (0.1mol) was added at 25 to Stir and react at 60°C for 2 hours, add 5 g of 30% hydrogen peroxide, continue the reaction for 6-10 hours, and the reaction is complete. The reaction solution was post-treated to obtain triglycidyl cyanurate with a yield of 82.1% and a melting point of 103-115°C; 1 H NMR (400MHz, CDCl 3 )δ: 2.71~2.85(m, 6H, 3×CH 2 ), 3.27~3.28(m, 3H, 3×CH), 4.08~4.16(m, 6H, 3×CH 2 ).
Embodiment 3
[0036] Preparation of triglycidyl cyanurate
[0037] Ethanol (50ml), sodium bicarbonate (0.05mol), triallyl isocyanurate (0.03mol), and acetonitrile (0.09mol) were stirred for 15 minutes, and 30% hydrogen peroxide (0.1mol) was added at 25 to Stir and react at 60°C for 2 hours, add 5 g of 30% hydrogen peroxide, and continue the reaction for 9 to 10 hours until the reaction is complete. The reaction solution was post-treated to obtain triglycidyl cyanurate with a yield of 81.2% and a melting point of 103-115°C; 1 H NMR (400MHz, CDCl 3 )δ: 2.71~2.85(m, 6H, 3×CH 2 ), 3.27~3.28(m, 3H, 3×CH), 4.08~4.16(m, 6H, 3×CH 2 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com