Moxifloxacin injection preparation
A technology of moxifloxacin and moxifloxacin hydrochloride, applied in the field of pharmaceutical preparations, can solve the problems of poor operability, high production cost, low sterility assurance level and the like, and achieve the effects of high safety and stable preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
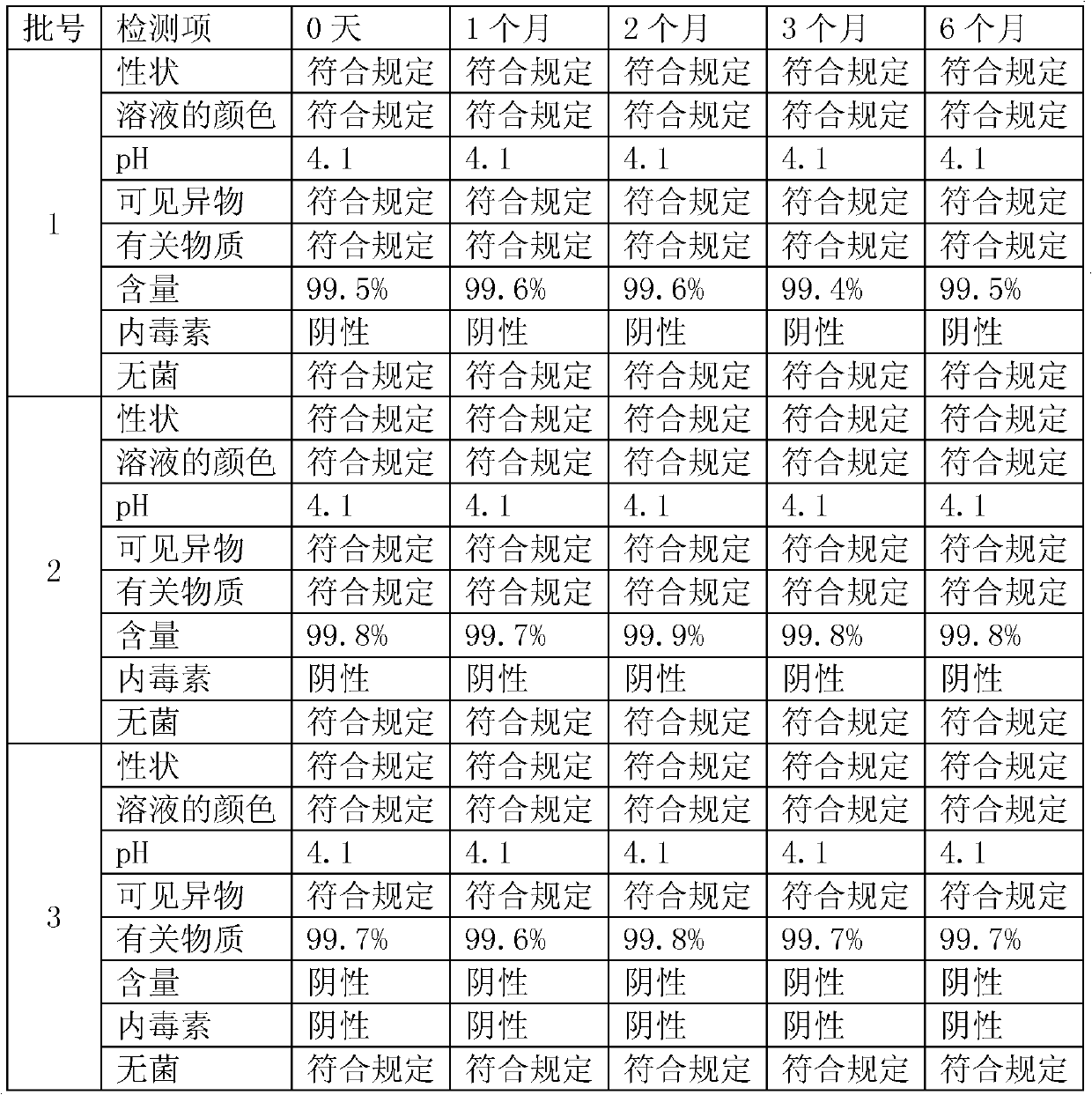
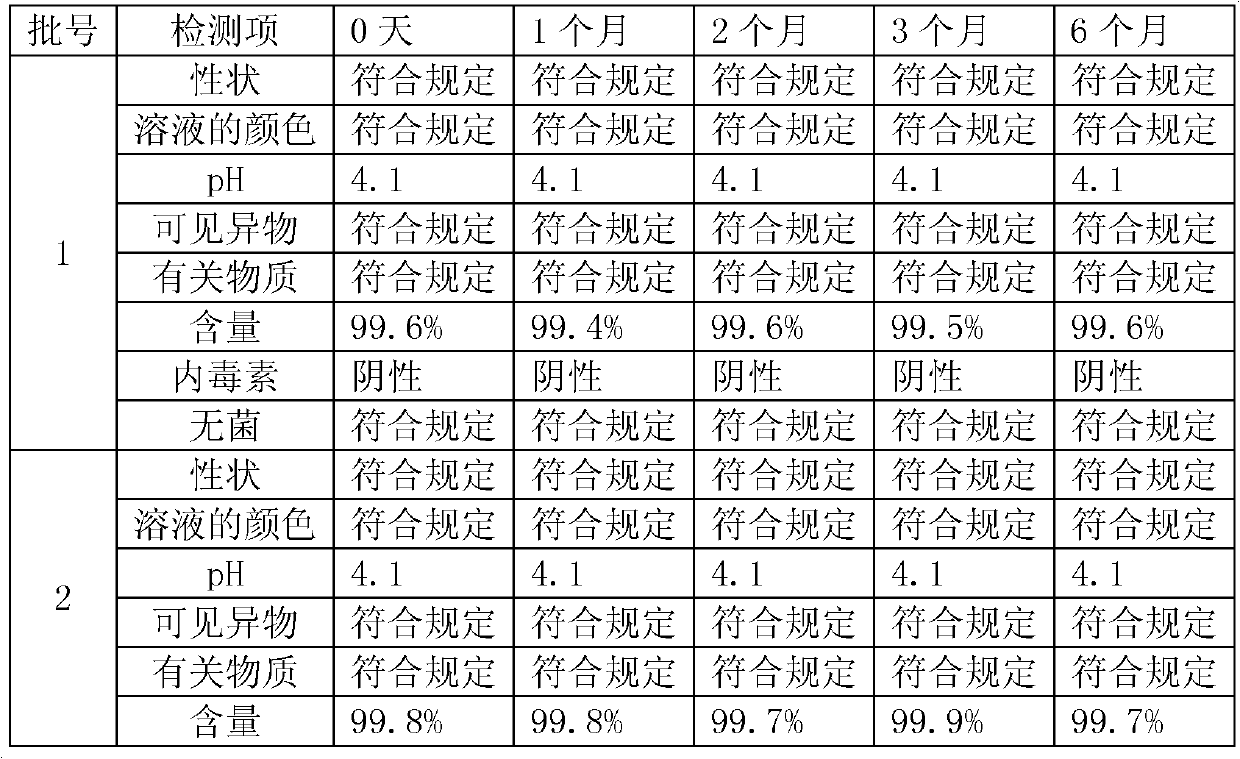
Embodiment 1
[0043] Embodiment 1, moxifloxacin hydrochloride injection
[0044]
[0045] Preparation:
[0046] (1) Take 90% of the prescribed amount of water, cool it to 60°C, weigh the prescribed amount of moxifloxacin hydrochloride, put it in a container and stir until it is completely dissolved.
[0047] (2) Take the test solution of (1) and adjust the pH to 4.0-4.5 with 0.1mol / L hydrochloric acid solution.
[0048] (3) Add 0.1% activated carbon (w / v) and keep warm for 30 minutes. The 5um titanium filter is decarburized once, the 0.22um water membrane is finely filtered twice, one membrane at a time, and the semi-finished product is inspected.
[0049] (4) Filling (20ml brown ampoule bottle), fusion sealing.
[0050] (5) Sterilize at 116°C for 30 minutes.
[0051] (6) Check Cheng.
[0052] (7) Packaging.
Embodiment 2
[0053] Embodiment 2,, moxifloxacin hydrochloride injection
[0054]
[0055] Preparation:
[0056] (1) Take 90% of the prescribed amount of water, cool it to 60°C, weigh the prescribed amount of moxifloxacin hydrochloride, put it in a container and stir until it is completely dissolved.
[0057] (2) Take the test solution of (1) and adjust the pH to 4.25 with 0.1mol / L hydrochloric acid solution.
[0058] (3) Add 0.1% activated carbon (w / v), keep warm for 30 minutes, decarburize the 5um titanium filter once, fine filter twice with the 0.22um water membrane, one membrane each time, and inspect the semi-finished product.
[0059] (4) Filling (20ml brown ampoule bottle), fusion sealing.
[0060] (5) Sterilize at 116°C for 30 minutes.
[0061] (6) Check Cheng.
[0062] (7) Packaging.
Embodiment 3
[0063] Embodiment 3, moxifloxacin hydrochloride injection
[0064]
[0065] Preparation:
[0066] (1) Take 90% of the prescribed amount of water, cool it to 60°C, weigh the prescribed amount of moxifloxacin hydrochloride, put it in a container and stir until it is completely dissolved.
[0067] (2) Take the test solution of (1) and adjust the pH to 4.25 with 0.1mol / L hydrochloric acid solution.
[0068] (3) Add 0.1% activated carbon (w / v), keep warm for 30 minutes, decarburize the 5um titanium filter once, fine filter twice with the 0.22um water membrane, one membrane each time, and inspect the semi-finished product.
[0069] (4) Filling (20ml brown ampoule bottle), fusion sealing.
[0070] (5) Sterilize at 116°C for 30 minutes.
[0071] (6) Check Cheng.
[0072] (7) Packaging.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com