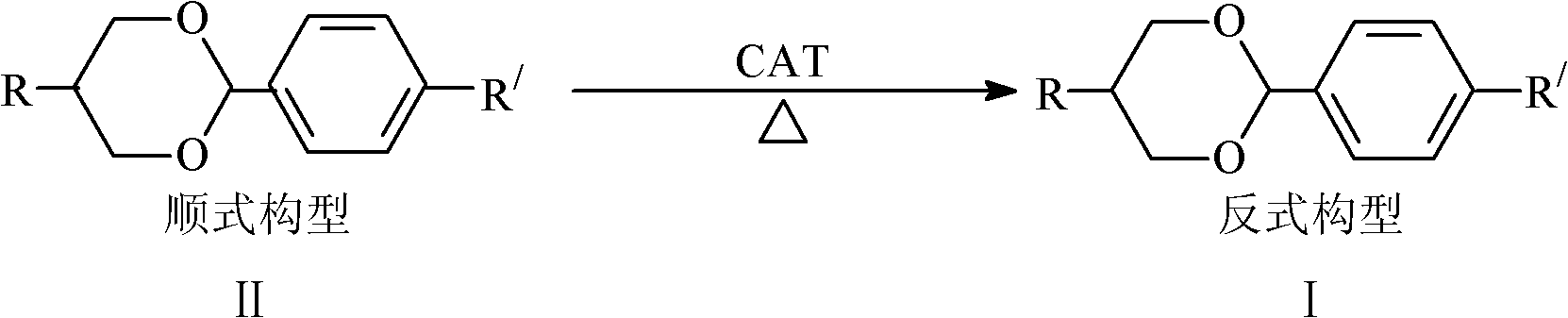
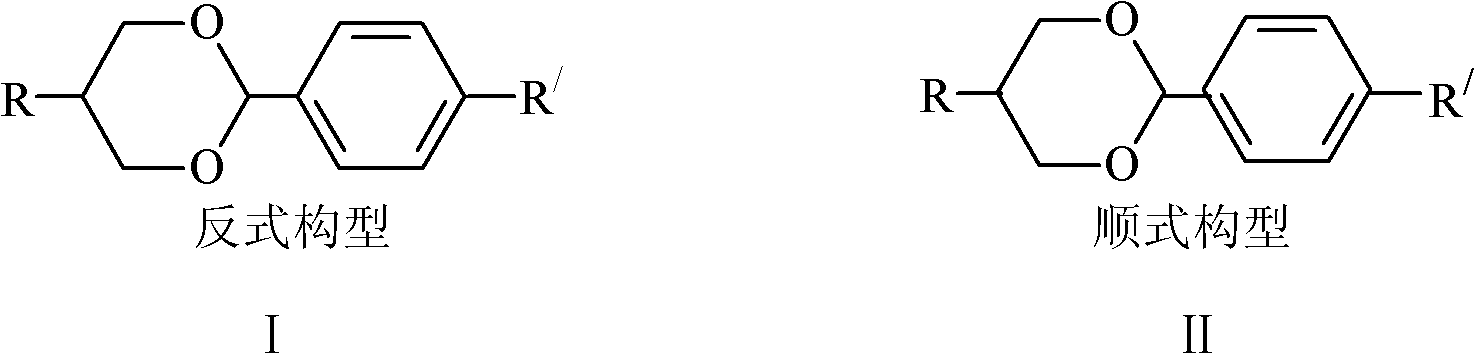Method for transforming cisconfiguration of oxane compounds to transconfiguration
A technology of cis-configuration and trans-configuration, applied in organic chemistry and other fields, can solve the problems of low yield of trans-oxane compounds and environmental pollution, so as to improve the utilization rate of raw materials, reduce environmental pollution, and improve utilization rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Embodiment 1 (preparation of trans 5-n-pentyl-2-(4-cyanophenyl)-1,3-dioxane (I a))
[0018] Add cis-5-n-pentyl-2-(4-cyanophenyl)-1,3-dioxane 20g successively in a 250mL three-necked reaction flask equipped with mechanical stirring, reflux condenser, and addition funnel, and the catalyst is Toluenesulfonic acid 2g, toluene 100mL, start stirring and heating, reflux reaction at 105-110°C for 3-8h, sample analysis, confirm the reaction is terminated according to the gas phase analysis results, cool, then add water 50mL to the reaction liquid, separate liquid, water phase Extract with 20 mL of toluene, combine the organic phases, wash with water until neutral, dry with 5 g of anhydrous magnesium sulfate for 8 h, filter, then rinse the filter cake twice with 10 mL of toluene, and take a sample analysis to determine the crude product quality ω (Ia): 76%, After distilling off the solvent, it was purified to obtain 13.9 g of product (Ia), ω(Ia): 99.5%, yield: 69.5%. Product mas...
Embodiment 2
[0019] Example 2 (preparation of trans 5-p-ethylphenyl-2-(4-cyanophenyl)-1,3-dioxane (I b))
[0020] Add cis-5-p-ethylphenyl-2-(4-cyanophenyl)-1,3-dioxane 20g successively in a 250mL three-necked reaction flask equipped with mechanical stirring, reflux condenser, and addition funnel, Catalyst p-toluenesulfonic acid 0.2g, toluene 100mL, start stirring and heating, reflux at 105-110°C for 3-8h, other conditions are the same as in Example 1, and purify to obtain 14.3g of product (I b), ω(I b): 99.5 %, yield: 71.5%. Product mass spectrometry result: ES-MSm / z: 293[M]+.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com