Method for synthesis of tyrosine derivative
A synthesis method and tyrosine technology are applied in the preparation of organic compounds, chemical instruments and methods, preparation of carboxylic acid amides, etc., which can solve the problem of low purity of the synthesis method and achieve the effect of good product quality.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
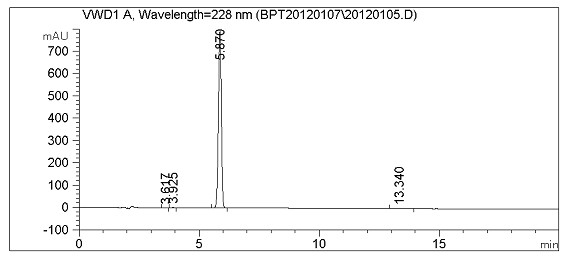
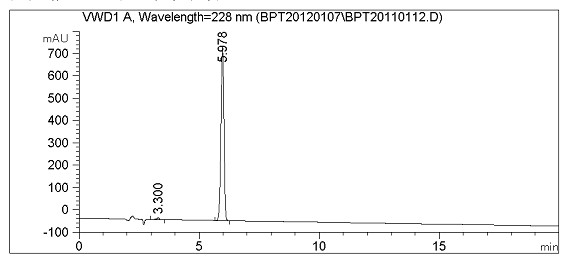
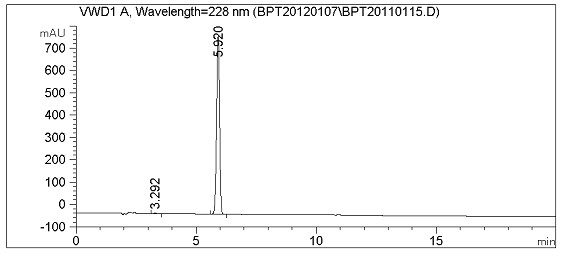
Image
Examples
Embodiment 1
[0022] O , N-(2- benzene Preparation of formyl)-L--tyrosine:
[0023] In a 1000ml four-necked reaction flask, add 500ml of purified water, 20g of sodium hydroxide, control the temperature to 20°C, add 20g of L-tyrosine, 35g of benzoyl chloride, control the temperature to 50°C, stir for 60 minutes, add acetone dropwise 200ml, stirred for 1 hour, cooled to 5°C, and 16g of concentrated hydrochloric acid was added dropwise. Stir for 1 hour, centrifuge, wash with 50ml of purified water to obtain about 90g of O,N-(2-benzoyl)-L--tyrosine wet product, and vacuum dry at 80°C for 10 hours to obtain dry O,N-(2 -Benzoyl)-L-tyrosine 41g (content 99%, K.F: 0.5%).
Embodiment 2
[0025] O , N-(2- benzene Preparation of formyl)-L--tyrosine:
[0026] In a 1000ml four-necked reaction flask, add 500ml of purified water, 28g of potassium hydroxide, control the temperature to 10°C, add 20g of L-tyrosine, 35g of benzoyl chloride, control the temperature to 40°C, stir for 60 minutes, add acetone dropwise 200ml, stirred for 1 hour, cooled to 5°C, and 16g of concentrated hydrochloric acid was added dropwise. Stir for 1 hour, centrifuge, wash with 50ml of purified water to obtain about 90g of O,N-(2-benzoyl)-L--tyrosine wet product, and vacuum dry at 80°C for 10 hours to obtain dry O,N-(2 -Benzoyl)-L-tyrosine 41.5g (content 99.1%, K.F: 0.4%).
Embodiment 3
[0028] Preparation of N-benzoyl-L-tyrosyl di-n-propylamine:
[0029] Add 300ml of ethyl acetate to a 1000ml four-necked bottle, cool down to 0°C, add 40g of O,N-(2-benzoyl)-L-tyrosine, dropwise add 22g of triethylamine, and stir for 30 minutes; 25g of ethyl chloroformate, stirred at 30°C for 30 minutes, added 33g of dipropylamine, raised the temperature to 40°C and stirred for 30 minutes, added 5g of concentrated hydrochloric acid and 100ml of water, stirred for 15 minutes, separated into layers, removed the water layer, added 30g of sodium chloride and 100ml of purified water, separate layers, remove the water layer, distill the organic layer under reduced pressure at 40°C until solids are precipitated and cool down to 0°C, add 300ml of methanol, 5g of sodium hydroxide, stir at 0°C for 1 hour, and use 30g of concentrated hydrochloric acid and 100ml of purified water Adjust pH=1.0~2.0, stir at 0°C for 1 hour. After centrifugation, 30 ml of purified water and 30 ml of methan...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com