Method for representing activity relationships of antibacterial proteins or polypeptides
An activity relationship, protein technology, applied in the field of amino acid structure characterization, can solve the problems of difficulty in collecting experimental parameters of non-coding amino acid diversity, difficult, and few research reports.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
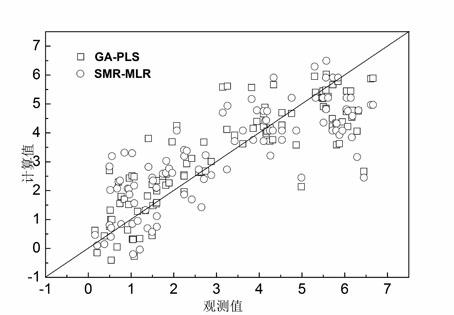
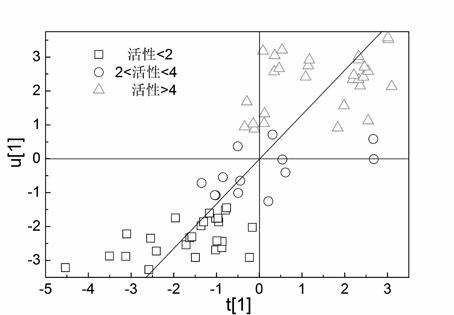
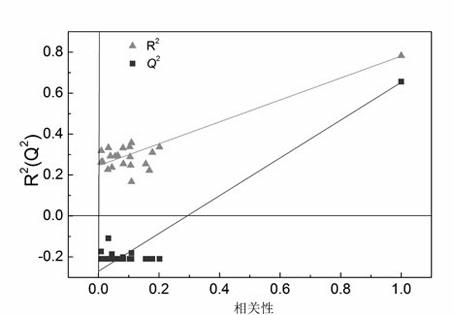
[0030] Example 1 QSAR research on 101 cationic antimicrobial peptides
[0031] The selected sample set is from Cherkasov et al. [26] (see Table 4.21 for the sources of 101 peptides and their antibacterial potency values). This group of compounds is derived from the hybrid polypeptide CAMEL0 which is respectively fused with the C and N terminal sequences of natural cecropin and melittin, and is rich in leucine (L) residues. Experiments have proved that CAMEL-s has high activity against various Gram-positive and negative bacterial strains. However, its exact mode of action mechanism is still unclear, so it is worthy of further study on the factors and mechanism affecting its antibacterial activity. Each AMP molecular structure was characterized by VSTPV descriptor. In order to verify the external predictive ability of the model, first sort 101 antimicrobial peptide samples according to the Y value (antibacterial potency) from high to low, then select samples 3, 6, 9... as t...
Embodiment 2
[0044] Example 2 Quantitative structure-activity relationship characterization of antibacterial activity of bovine lactoferrin hydrolyzed peptide
[0045] Bovine Lactoferrin (LFB) is an iron-binding glycoprotein with a relative molecular mass of about 80kD, which mainly exists in various exocrine substances such as milk, tears, and saliva of mammals. LBF has a variety of unique biological functions such as enhancing iron transmission and absorption, broad-spectrum antibacterial properties, immune regulation, anti-oxidation, promoting the balance of intestinal flora, growth factors, anti-inflammation, anti-virus, and anti-cancer effects [27-29] . It has been applied to the development and prevention of human immunodeficiency virus (HIV), macrophage virus (CMV), human herpes simplex virus (HSV), hepatitis C virus (HCV), Helicobacter pylori and other drugs and immune enhancers and baby food [30] .
[0046] Three peptides are obtained after lactoferrin is hydrolyzed, and the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com