Precipitated iron catalyst for Fischer-Tropsch synthesis and preparation method thereof
A Fischer-Tropsch synthesis and catalyst technology, which is used in catalyst activation/preparation, chemical instruments and methods, preparation of liquid hydrocarbon mixtures, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
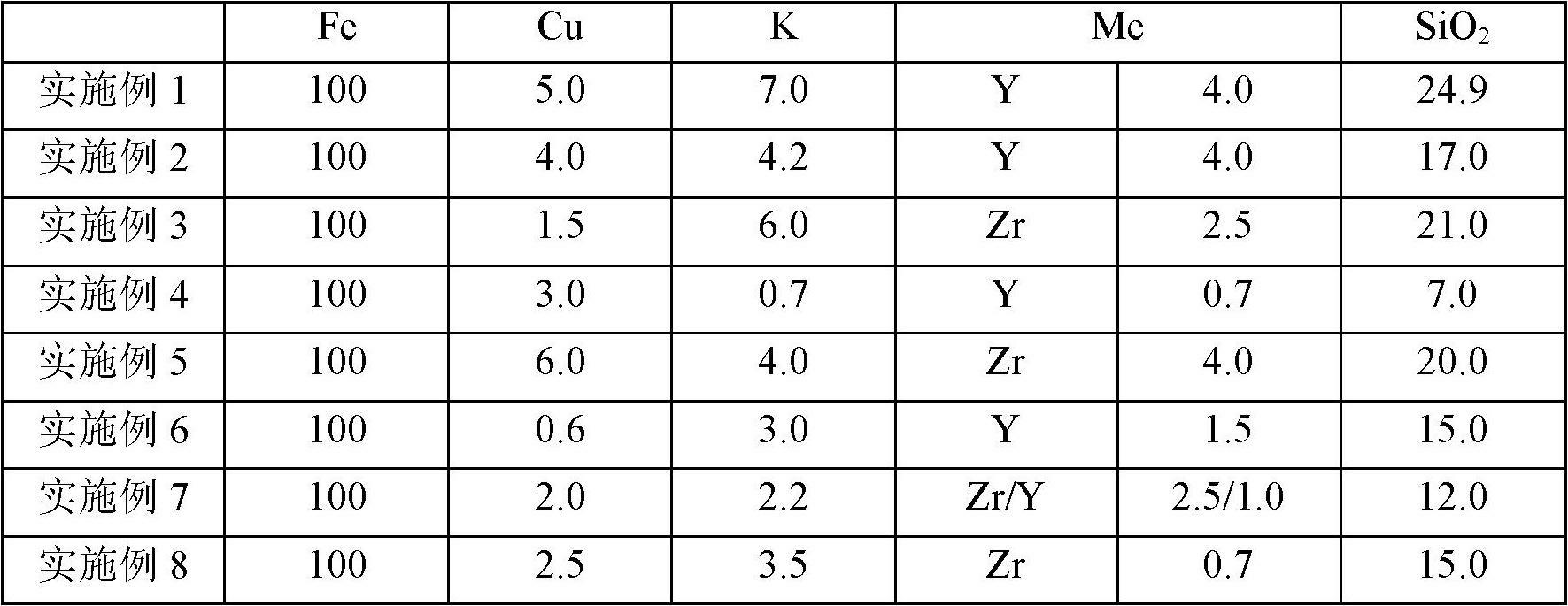
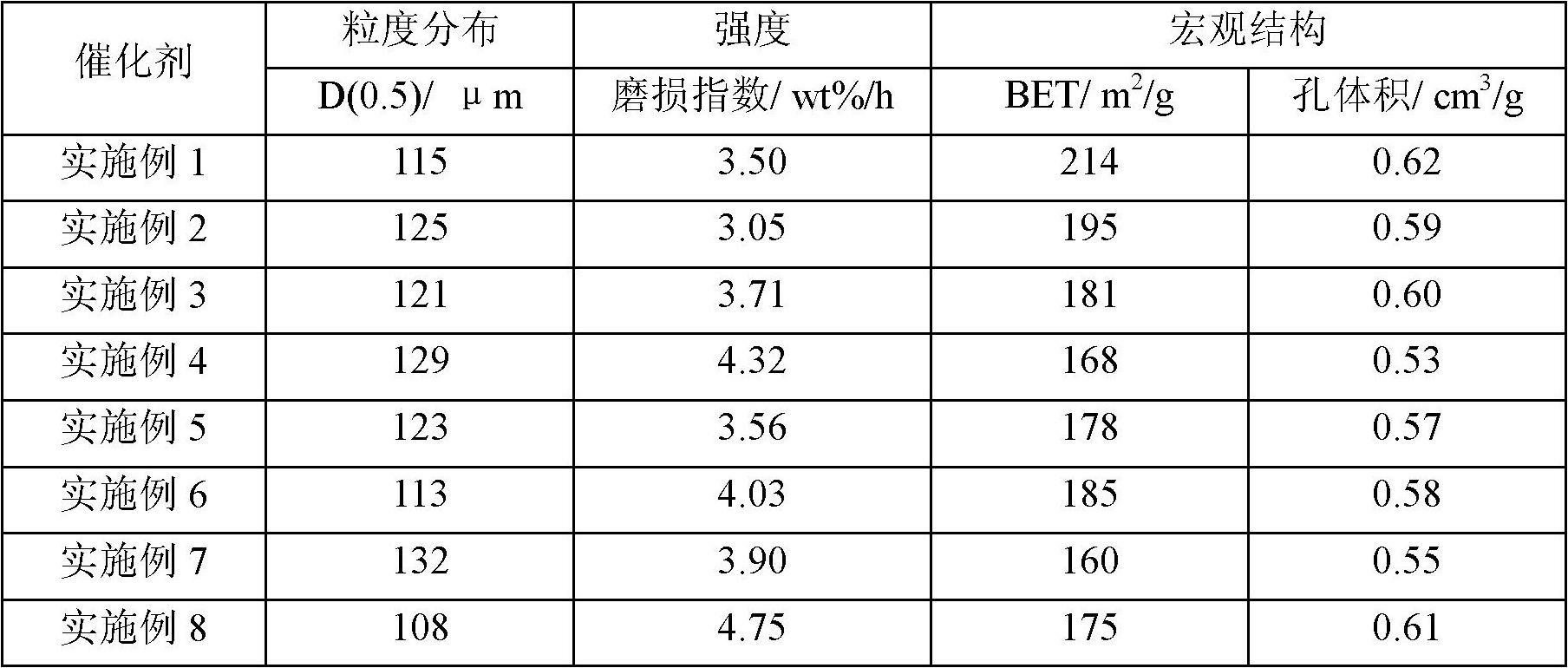
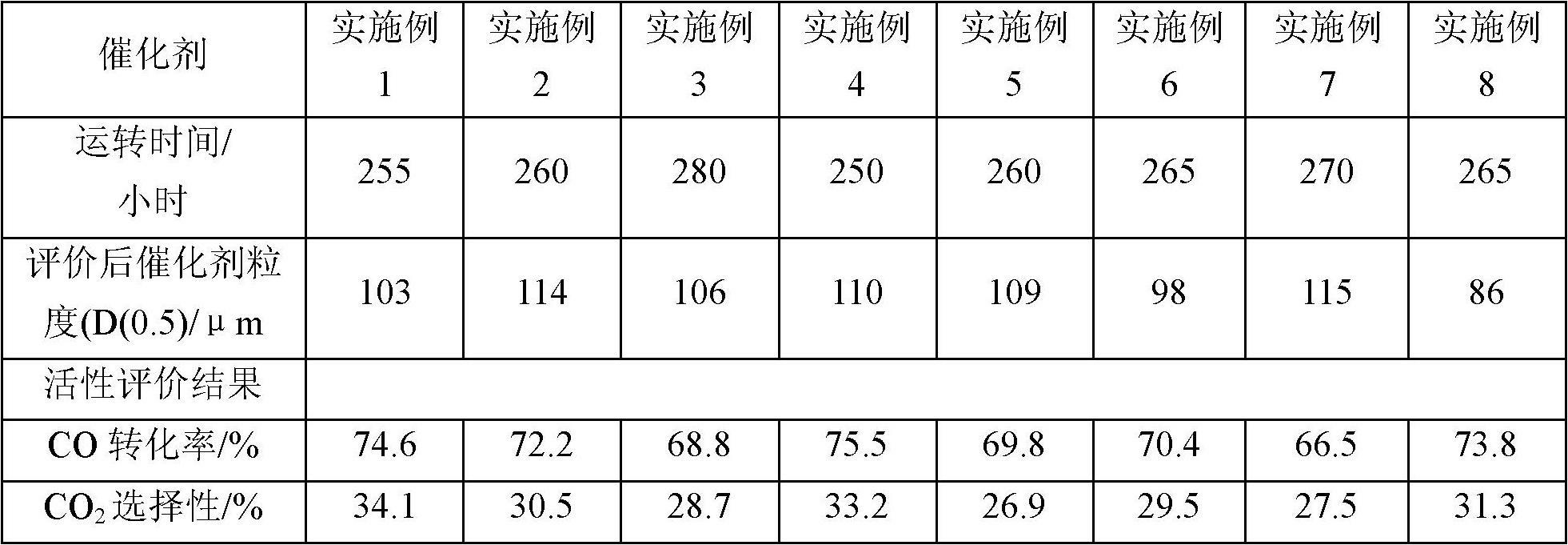
[0035] According to a typical implementation of the present invention, the preparation method of the above-mentioned catalyst includes the following steps: 1) mixing the nitrate or oxide of Fe, the nitrate or oxide of Me and the binding agent to obtain the first mixed solution, the first The mixed solution is co-precipitated with ammonium carbonate or ammonia water to obtain a precipitation slurry, wherein Me is a transition metal element Zr and / or Y, the binder is an aqueous solution of a silicon compound, and the binder is introduced into the second SiO in a mixed solution 2 The mass ratio to Fe is 0.01 to 4.9:100; 2) After the precipitation slurry is aged for a certain period of time at the same temperature as the precipitation reaction, it is transferred to a suction filtration device for suction filtration and washing to obtain a filter cake, which is added to the filter cake Deionized water for repulping and reintroducing SiO 2 , K and Cu additives to obtain the second ...
Embodiment 1
[0070] 1) Weigh 13.0Kg Fe(NO 3 ) 3 9H 2 O, 0.09Kg Y 2 o 3 , add 50L deionized water and stir to dissolve. Adding 1.20Kg mass concentration is the K of 15wt% in this solution that is stirring again. 2 SiO 3 aqueous solution, fully stirred to obtain the first mixed solution. Weigh 8.5Kg (NH 4 ) 2 CO 3 , Add 30.0L deionized water and stir to dissolve to obtain a precipitated slurry. The iron salt mixed solution is heated to above 65° C. by means of jacket heating, and then flows into a vigorously stirred reaction tank with the heated precipitant solution to produce co-precipitation. Control the precipitation temperature in the reaction tank at 65°C and the pH at 7.5 by adjusting the acid and alkali salt delivery pumps respectively.
[0071] 2) After the precipitation is completed, age the precipitated slurry at the same temperature for 30 minutes, transfer it to a suction filtration device for suction filtration within 8 minutes, discard the filtrate and wash the filte...
Embodiment 2
[0077] 1) Prepare co-precipitation filter cake with the same method described in Example 1.
[0078] 2) Repulping with deionized water with a filter cake:water mass ratio of 7.5:1. Add 1.16kg of binder 2 solution and 0.21kg of 45% KOH aqueous solution into the slurry while stirring, keep the system temperature at about 70°C, and stir under high shear for 25min. Then add 0.47Kg 40wt% Cu(NO 3 ) 2 aqueous solution, and continued high-shear stirring for 30 minutes to obtain the second mixed solution, at which point the pH value of the system obtained from the second mixed solution would be greatly reduced from the original value. However, if the pH value of the copper nitrate solution is still not lower than 4.5 after all the copper nitrate solution is poured out, the pH value can be adjusted to about 4.5 with additional dilute nitric acid. Stirring was continued for 60 min at the above system temperature. Finally, a catalyst slurry with a total solid content of about 26 wt % ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com