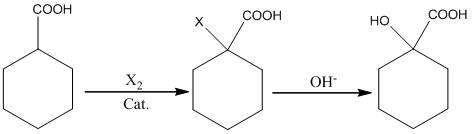
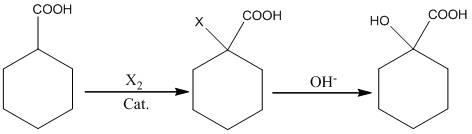
Preparation method of alpha-hydroxy-cyclohexanecarboxylic acid
A technology of hydroxycyclohexyl and cyclohexyl formic acid, which is applied in the field of preparation of pharmaceutical intermediates, can solve the problems of extremely demanding process requirements for waste acid treatment, potential safety hazards, safety accidents, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Add 200g (1mol) of 20% sodium hydroxide solution dropwise to the above system, raise the temperature of the system to 85-90°C, keep it warm for 1h, cool to room temperature, adjust the pH of the system to 2-3 with 20% hydrochloric acid, and then extract it with 200ml of toluene After concentration and crystallization, 36g of the product was obtained. The yield calculated with cyclohexyl formic acid was 70%.
[0016] Embodiment two
Embodiment 2
[0018] Add 120g (0.6mol) of 20% sodium hydroxide solution dropwise to the above system, raise the temperature of the system to 60-70°C, keep it warm for 1h, cool to room temperature, adjust the pH of the system to 2-3 with 20% sulfuric acid, and then extract with 150ml of toluene 2 times, after concentration and crystallization, 31g of the product was obtained. The yield calculated with cyclohexyl formic acid was 72%.
[0019] Embodiment three
Embodiment 3
[0021] Add 318g (0.3mol) of 10% sodium carbonate solution dropwise to the above system, control the temperature of the system at 20-40°C, keep it warm for 2 hours, cool to room temperature, adjust the pH of the system to 2-3 with 20% sulfuric acid, and then extract twice with 150ml of toluene After concentration and crystallization, 26g of the product was obtained. The yield is 60% based on cyclohexyl formic acid.
[0022] Embodiment four
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com