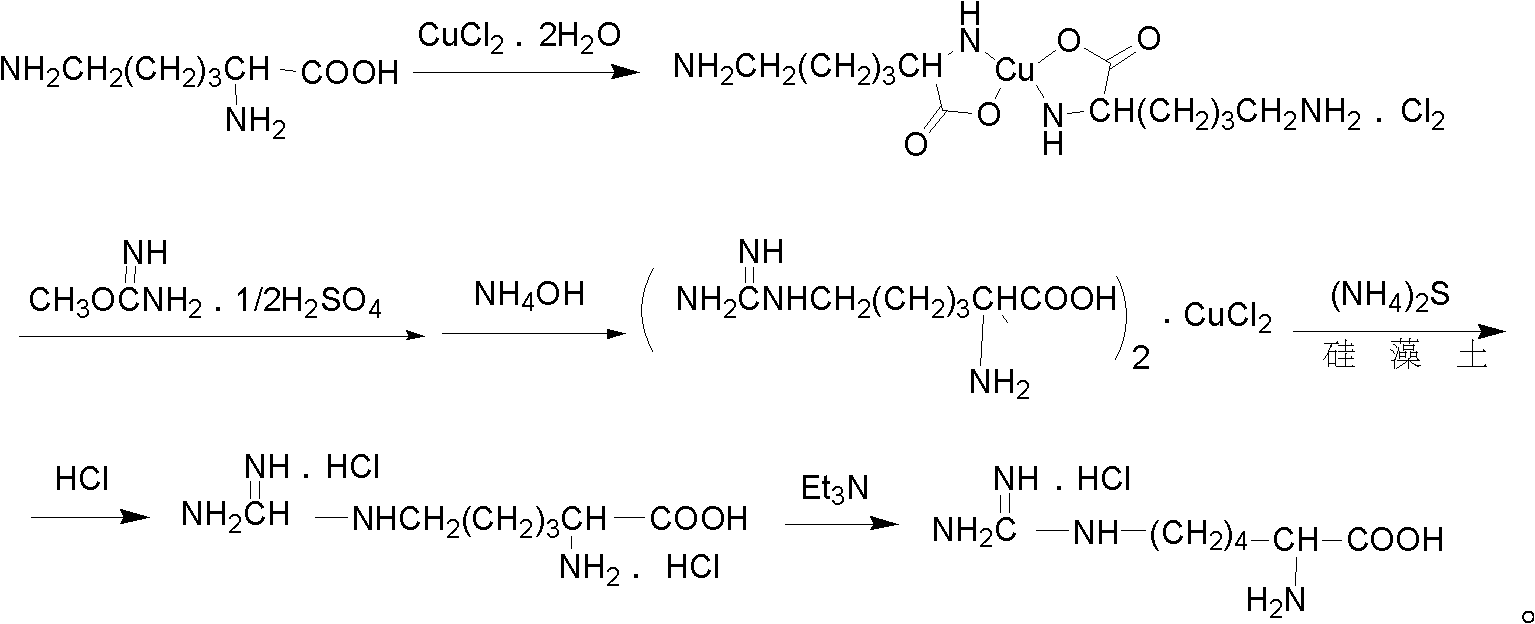Preparation method of L-homoarginine hydrochloride
A technology of homoarginine hydrochloride and lysine, applied in the field of preparation of L-homoarginine hydrochloride, which can solve the problems of inconvenient large-scale production, long reaction time, complicated post-treatment, etc. , achieve the effect of shortening the reaction time, high reaction yield and reducing production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0011] The preparation method of the L-homoarginine hydrochloride provided in this embodiment comprises the following steps:
[0012] 1. Weigh 44.6g of L-lysine, add 150mL of water to dissolve, and prepare L-lysine solution, and dissolve 26.3g of CuCl 2 2H 2 O was added to the L-lysine solution, and stirred at 20°C for 1.5 hours to obtain a reaction solution A, which was blue in color;
[0013] 2. Weigh 45.8g of O-methylisourea hemisulfate, dissolve it in 100mL of NaOH solution with a mass percentage concentration of 10%, and then add it to the reaction solution A to obtain the reaction solution B, adjust the reaction solution B with 60mL of ammonia water When the pH value reaches 10.60, the blue color becomes lighter;
[0014] 3. Stir the pH-adjusted reaction solution B at 20°C for 24 hours, and then put it in the fresh-keeping layer of the refrigerator (at a temperature of 3°C) for 12 hours. A blue slurry precipitates out, and then quickly filters it to obtain the The sol...
Embodiment 2
[0018] The preparation method of the L-homoarginine hydrochloride provided in this embodiment comprises the following steps:
[0019] 1. Weigh 300g of L-lysine, add 1L of water to dissolve it, and make L-lysine solution, mix 180g of CuCl 22H 2 O was added to the L-lysine solution, and stirred at 30°C for 2.5 hours to obtain a reaction solution A, which was blue in color;
[0020] 2. Weigh 308g of O-methylisourea hemisulfate, dissolve it in a NaOH solution with a concentration of 10% by mass, then add it to the reaction solution A to obtain the reaction solution B, adjust the pH of the reaction solution B with 320mL of ammonia water When the value reaches 10.60, the blue becomes lighter;
[0021] 3. Stir the pH-adjusted reaction solution B at 30°C for 24 hours, then put it into the fresh-keeping layer of the refrigerator (at a temperature of 3°C) for 12 hours, a blue slurry precipitates out, and then quickly filters it to obtain the The solid was dried in a vacuum oven for 3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com