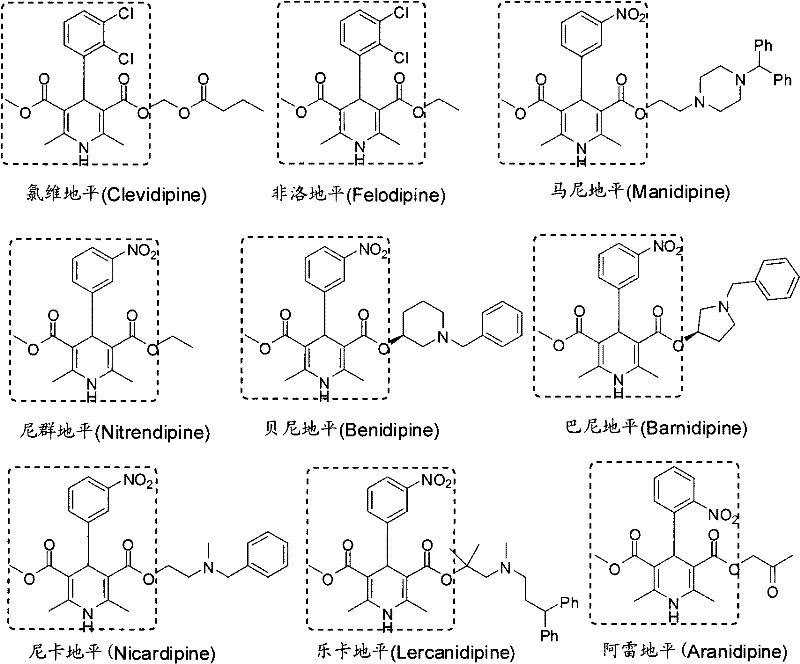
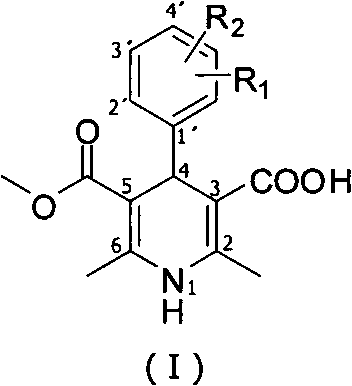
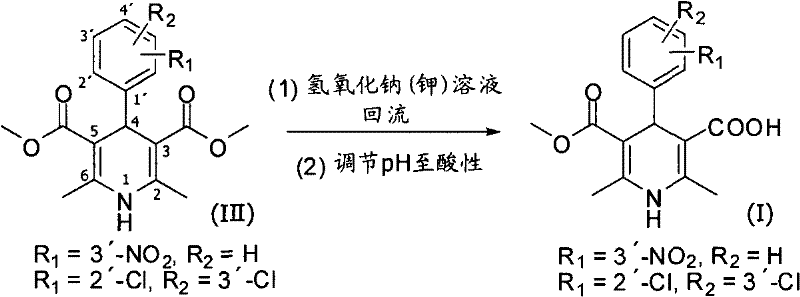
Method for preparing key intermediate of dihydropyridines calcium channel antagonist and for synthesizing clevidipine butyrate
A kind of technology of clevidipine butyrate and methyl butyrate, applied in the direction of organic chemistry and the like, can solve problems such as unfavorable industrial production, increase the difficulty of synthesis process and operation steps, increase the operation route of synthesizing "dipine" compounds, etc. Avoid acidic chemical raw materials, reduce post-processing operations, and facilitate separation and purification.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1: Preparation of 4-(2,3-dichlorophenyl)-2,6-dimethyl-1,4-dihydro-5-methoxycarbonyl-3-pyridinecarboxylic acid:
[0037] In a 20L reactor, add 2,3-dichlorobenzaldehyde (1.75kg, 10mol), tert-butyl acetoacetate (1.74kg, 11mol) and 3-amino-2-butenoic acid methyl ester (1.15kg, 10mol) , ethanol as the reaction solvent, reacted at 70°C for 18 hours, concentrated under reduced pressure to remove the reaction solvent, and obtained the crude product of Hantsch cyclization. The crude product was recrystallized from 95% ethanol to give 4-(2,3-dichlorophenyl)-2,6-dimethyl-1,4-dihydro-5-methoxycarbonyl-3-pyridine as a light yellow solid Pure tert-butyl carboxylate (3.2kg, yield 78%). 1 H NMR (600MHz, CDCl 3 ): δ7.29~7.24(m, 2H), 7.08~7.05(m, 1H), 5.56(s, 1H), 5.41(s, 1H), 3.60(s, 3H), 2.27(s, 3H), 2.24(s, 3H), 1.37(s, 9H); 13 C NMR (150MHz, CDCl 3 ): δ168.0, 166.9, 147.3, 144.3, 142.2, 132.9, 131.0, 130.1, 128.2, 126.7, 105.2, 102.5, 80.3, 50.8, 39.6, 28.3, 19.5, 19.4. ...
Embodiment 2
[0039] Example 2: Preparation of 4-(2-nitrophenyl)-2,6-dimethyl-1,4-dihydro-5-methoxycarbonyl-3-pyridinecarboxylic acid:
[0040] In 20L reactor, add 2-nitrobenzaldehyde (1.51kg, 10mol), tert-butyl acetoacetate (1.74kg, 11mol) and 3-amino-2-butenoic acid methyl ester (1.15kg, 10mol), ethanol As a reaction solvent, react at 70° C. for 24 hours, and concentrate under reduced pressure to remove the reaction solvent to obtain a crude product of Hantsch cyclization. The crude product was recrystallized from 95% ethanol to give 4-(2-nitrophenyl)-2,6-dimethyl-1,4-dihydro-5-methoxycarbonyl-3-pyridinecarboxylic acid as a pale yellow solid Pure tert-butyl ester (3.182kg, yield 82%).
[0041] The above product (3.182kg) was dissolved in tetrahydrofuran, and trifluoroacetic acid (600ml, 8.2mol) was added dropwise at 0°C, slowly raised to room temperature and reacted for 2 hours, then a large amount of ice water was added, and a large amount of solid precipitated out. The crude product o...
Embodiment 3
[0042] Example 3: Preparation of 4-(3-nitrophenyl)-2,6-dimethyl-1,4-dihydro-5-methoxycarbonyl-3-pyridinecarboxylic acid:
[0043] In 20L reactor, add 3-nitrobenzaldehyde (1.51kg, 10mol), tert-butyl acetoacetate (1.74kg, 11mol) and 3-amino-2-butenoic acid methyl ester (1.15kg, 10mol), ethanol As a reaction solvent, react at 70° C. for 24 hours, and concentrate under reduced pressure to remove the reaction solvent to obtain a crude product of Hantsch cyclization. The crude product was recrystallized from 95% ethanol to give 4-(3-nitrophenyl)-2,6-dimethyl-1,4-dihydro-5-methoxycarbonyl-3-pyridinecarboxylic acid as a pale yellow solid Pure tert-butyl ester (2.9kg, yield 75%).
[0044] The above product (2.9kg) was dissolved in tetrahydrofuran, and concentrated sulfuric acid (400ml, 7.5mol) was added dropwise at 0°C, slowly raised to room temperature and reacted for 2 hours, then a large amount of ice water was added, and a large amount of solid precipitated out, filtered The crud...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com